Cross-electrophile coupling: principles of reactivity and selectivity
- PMID: 24820397
- PMCID: PMC4049235
- DOI: 10.1021/jo500507s
Cross-electrophile coupling: principles of reactivity and selectivity
Abstract
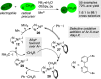
A critical overview of the catalytic joining of two different electrophiles, cross-electrophile coupling (XEC), is presented with an emphasis on the central challenge of cross-selectivity. Recent synthetic advances and mechanistic studies have shed light on four possible methods for overcoming this challenge: (1) employing an excess of one reagent; (2) electronic differentiation of starting materials; (3) catalyst-substrate steric matching; and (4) radical chain processes. Each method is described using examples from the recent literature.
Figures







References
-
- Negishi E.-i.; Meijere A. d.. Handbook of Organopalladium Chemistry for Organic Synthesis, 1st ed.; Wiley-VCH Verlag: Weinheim, 2002; Vols. 1 and 2, pp 1–3424.
- Meijere A. d.; Diederich F.. Metal-Catalyzed Cross-Coupling Reactions, 2nded.;Wiley-VCH: Weinheim, 2004; pp 1–938.
-
- Molander G. A.; Jean-Gérard L. Org. React. 2013, 79, 1–316.
- Gillis E. P.; Burke M. D. J. Am. Chem. Soc. 2007, 129, 6716–6717. - PubMed
- Noguchi H.; Hojo K.; Suginome M. J. Am. Chem. Soc. 2007, 129, 758–759. - PubMed
- Nakao Y.; Chen J.; Tanaka M.; Hiyama T. J. Am. Chem. Soc. 2007, 129, 11694–11695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

