Polymeric stent materials dysregulate macrophage and endothelial cell functions: implications for coronary artery stent
- PMID: 24820736
- PMCID: PMC4070878
- DOI: 10.1016/j.ijcard.2014.04.228
Polymeric stent materials dysregulate macrophage and endothelial cell functions: implications for coronary artery stent
Abstract
Background: Biodegradable polymers have been applied as bulk or coating materials for coronary artery stents. The degradation of polymers, however, could induce endothelial dysfunction and aggravate neointimal formation. Here we use polymeric microparticles to simulate and demonstrate the effects of degraded stent materials on phagocytic activity, cell death and dysfunction of macrophages and endothelial cells.
Methods: Microparticles made of low molecular weight polyesters were incubated with human macrophages and coronary artery endothelial cells (ECs). Microparticle-induced phagocytosis, cytotoxicity, apoptosis, cytokine release and surface marker expression were determined by immunostaining or ELISA. Elastase expression was analyzed by ELISA and the elastase-mediated polymer degradation was assessed by mass spectrometry.
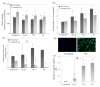
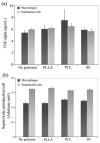
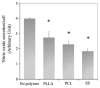
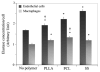
Results: We demonstrated that poly(D,L-lactic acid) (PLLA) and polycaprolactone (PCL) microparticles induced cytotoxicity in macrophages and ECs, partially through cell apoptosis. The particle treatment alleviated EC phagocytosis, as opposed to macrophages, but enhanced the expression of vascular cell adhesion molecule (VCAM)-1 along with decreased nitric oxide production, indicating that ECs were activated and lost their capacity to maintain homeostasis. The activation of both cell types induced the release of elastase or elastase-like protease, which further accelerated polymer degradation.
Conclusions: This study revealed that low molecule weight PLLA and PCL microparticles increased cytotoxicity and dysregulated endothelial cell function, which in turn enhanced elastase release and polymer degradation. These indicate that polymer or polymer-coated stents impose a risk of endothelial dysfunction after deployment which can potentially lead to delayed endothelialization, neointimal hyperplasia and late thrombosis.
Keywords: Dysfunction; Elastase; Endothelial cell; Poly(L-lactic acid); Polycaprolactone; Stent.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Figures

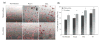
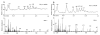
Similar articles
-
Effect of inflammation on endothelial cells induced by poly-L-lactic acid degradation in vitro and in vivo.J Biomater Sci Polym Ed. 2018 Oct;29(15):1909-1919. doi: 10.1080/09205063.2018.1517858. Epub 2018 Oct 8. J Biomater Sci Polym Ed. 2018. PMID: 30173602
-
Secreted Matrix Metalloproteinase-9 of Proliferating Smooth Muscle Cells as a Trigger for Drug Release from Stent Surface Polymers in Coronary Arteries.Mol Pharm. 2016 Jul 5;13(7):2290-300. doi: 10.1021/acs.molpharmaceut.6b00033. Epub 2016 Jun 9. Mol Pharm. 2016. PMID: 27241028
-
Surface Modification of Biodegradable Polymers towards Better Biocompatibility and Lower Thrombogenicity.PLoS One. 2015 Dec 7;10(12):e0142075. doi: 10.1371/journal.pone.0142075. eCollection 2015. PLoS One. 2015. PMID: 26641662 Free PMC article.
-
High-Density Lipoproteins and Apolipoprotein A-I Improve Stent Biocompatibility.Arterioscler Thromb Vasc Biol. 2018 Aug;38(8):1691-1701. doi: 10.1161/ATVBAHA.118.310788. Arterioscler Thromb Vasc Biol. 2018. PMID: 29954755 Review.
-
Antithrombotic stent coatings: hirudin/iloprost combination.Semin Interv Cardiol. 1998 Sep-Dec;3(3-4):177-83. Semin Interv Cardiol. 1998. PMID: 10406690 Review.
Cited by
-
Safety of terminally gamma-ray-sterilized screws coated with fibroblast growth factor 2-calcium phosphate composite layers in non-human primates.J Artif Organs. 2023 Sep;26(3):192-202. doi: 10.1007/s10047-022-01352-1. Epub 2022 Aug 8. J Artif Organs. 2023. PMID: 35941264
-
Characterization of Photoluminescent Polylactone-Based Nanoparticles for Their Applications in Cardiovascular Diseases.Front Bioeng Biotechnol. 2019 Nov 22;7:353. doi: 10.3389/fbioe.2019.00353. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31824940 Free PMC article.
-
Feasibility of Application of the Newly Developed Nano-Biomaterial, β-TCP/PDLLA, in Maxillofacial Reconstructive Surgery: A Pilot Rat Study.Nanomaterials (Basel). 2021 Jan 25;11(2):303. doi: 10.3390/nano11020303. Nanomaterials (Basel). 2021. PMID: 33503931 Free PMC article.
-
Curcumin attenuates inflammation of Macrophage-derived foam cells treated with Poly-L-lactic acid degradation via PPARγ signaling pathway.J Mater Sci Mater Med. 2022 Mar 18;33(4):33. doi: 10.1007/s10856-022-06654-7. J Mater Sci Mater Med. 2022. PMID: 35303193 Free PMC article.
-
Durable-polymer versus biodegradable-polymer drug-eluting stents in acute coronary syndromes: three-year outcomes of the HOST REDUCE POLYTECH RCT Trial.EuroIntervention. 2024 Jun 17;20(12):e750-e759. doi: 10.4244/EIJ-D-23-01053. EuroIntervention. 2024. PMID: 38887886 Free PMC article. Clinical Trial.
References
-
- Minami Y, Kaneda H, Inoue M, Ikutomi M, Morita T, Nakajima T. Endothelial dysfunction following drug-eluting stent implantation: a systematic review of the literature. Int J Cardiol. 2013;165:222–8. - PubMed
-
- Hofma SH, van der Giessen WJ, van Dalen BM, Lemos PA, McFadden EP, Sianos G, et al. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:166–70. - PubMed
-
- Jimenez-Valero S, Moreno R, Sanchez-Recalde A. Very Late Drug-Eluting Stent Thrombosis Related to Incomplete Stent Endothelialization: In-Vivo Demonstration by Optical Coherence Tomography. Journal of Invasive Cardiology. 2009;21:488–90. - PubMed
-
- Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P60 DK020593/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- 1UH2 TR000491/TR/NCATS NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R21 HL091465/HL/NHLBI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- UH2 TR000491/TR/NCATS NIH HHS/United States
- UH3 TR000491/TR/NCATS NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- HL091465/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

