Enhancing the quality of H/D exchange measurements with mass spectrometry detection in disulfide-rich proteins using electron capture dissociation
- PMID: 24820935
- PMCID: PMC4051250
- DOI: 10.1021/ac500904p
Enhancing the quality of H/D exchange measurements with mass spectrometry detection in disulfide-rich proteins using electron capture dissociation
Abstract
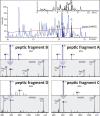
Hydrogen/deuterium exchange (HDX) mass spectrometry (MS) has become a potent technique to probe higher-order structures, dynamics, and interactions of proteins. While the range of proteins amenable to interrogation by HDX MS continues to expand at an accelerating pace, there are still a few classes of proteins whose analysis with this technique remains challenging. Disulfide-rich proteins constitute one of such groups: since the reduction of thiol-thiol bonds must be carried out under suboptimal conditions (to minimize the back-exchange), it frequently results in incomplete dissociation of disulfide bridges prior to MS analysis, leading to a loss of signal, inadequate sequence coverage, and a dramatic increase in the difficulty of data analysis. In this work, the dissociation of disulfide-linked peptide dimers produced by peptic digestion of the 80 kDa glycoprotein transferrin in the course of HDX MS experiments is carried out using electron capture dissociation (ECD). ECD results in efficient cleavage of the thiol-thiol bonds in the gas phase on the fast LC time scale and allows the deuterium content of the monomeric constituents of the peptide dimers to be measured individually. The measurements appear to be unaffected by hydrogen scrambling, even when high collisional energies are utilized. This technique will benefit HDX MS measurements for any protein that contains one or more disulfides and the potential gain in sequence coverage and spatial resolution would increase with disulfide bond number.
Figures

Similar articles
-
Measuring the hydrogen/deuterium exchange of proteins at high spatial resolution by mass spectrometry: overcoming gas-phase hydrogen/deuterium scrambling.Acc Chem Res. 2014 Oct 21;47(10):3018-27. doi: 10.1021/ar500194w. Epub 2014 Aug 29. Acc Chem Res. 2014. PMID: 25171396 Review.
-
Approach to characterization of the higher order structure of disulfide-containing proteins using hydrogen/deuterium exchange and top-down mass spectrometry.Anal Chem. 2014 Aug 5;86(15):7293-8. doi: 10.1021/ac501789e. Epub 2014 Jul 11. Anal Chem. 2014. PMID: 24988145 Free PMC article.
-
Hydrogen/Deuterium Exchange Mass Spectrometry with Integrated Electrochemical Reduction and Microchip-Enabled Deglycosylation for Epitope Mapping of Heavily Glycosylated and Disulfide-Bonded Proteins.Anal Chem. 2021 Dec 14;93(49):16330-16340. doi: 10.1021/acs.analchem.1c01728. Epub 2021 Nov 29. Anal Chem. 2021. PMID: 34843209
-
Regio-Selective Intramolecular Hydrogen/Deuterium Exchange in Gas-Phase Electron Transfer Dissociation.J Am Soc Mass Spectrom. 2017 May;28(5):971-977. doi: 10.1007/s13361-017-1612-4. Epub 2017 Feb 13. J Am Soc Mass Spectrom. 2017. PMID: 28194737
-
Hydrogen exchange mass spectrometry for studying protein structure and dynamics.Chem Soc Rev. 2011 Mar;40(3):1224-34. doi: 10.1039/c0cs00113a. Epub 2010 Dec 21. Chem Soc Rev. 2011. PMID: 21173980 Review.
Cited by
-
Construction of a Dual Protease Column, Subzero (-30 °C) Chromatography System and Multi-channel Precision Temperature Controller for Hydrogen-Deuterium Exchange Mass Spectrometry.J Res Natl Inst Stand Technol. 2020 Aug 12;vol:125025. doi: 10.6028/jres.125.025. eCollection 2020. J Res Natl Inst Stand Technol. 2020. PMID: 35573859 Free PMC article.
-
Mass Spectrometry Investigation of Some ATP-Binding Cassette (ABC) Proteins.Medicina (Kaunas). 2024 Jan 24;60(2):200. doi: 10.3390/medicina60020200. Medicina (Kaunas). 2024. PMID: 38399488 Free PMC article. Review.
-
Applications of hydrogen/deuterium exchange MS from 2012 to 2014.Anal Chem. 2015 Jan 6;87(1):99-118. doi: 10.1021/ac5040242. Epub 2014 Nov 14. Anal Chem. 2015. PMID: 25398026 Free PMC article. Review. No abstract available.
-
Fundamentals of HDX-MS.Essays Biochem. 2023 Mar 29;67(2):301-314. doi: 10.1042/EBC20220111. Essays Biochem. 2023. PMID: 36251047 Free PMC article. Review.
-
ROR and RYK extracellular region structures suggest that receptor tyrosine kinases have distinct WNT-recognition modes.Cell Rep. 2021 Oct 19;37(3):109834. doi: 10.1016/j.celrep.2021.109834. Cell Rep. 2021. PMID: 34686333 Free PMC article.
References
-
- Konermann L.; Pan J.; Liu Y.-H. Chem. Soc. Rev. 2011, 40, 1224–1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

