Functional plasticity in childhood brain disorders: when, what, how, and whom to assess
- PMID: 24821533
- PMCID: PMC4231018
- DOI: 10.1007/s11065-014-9261-x
Functional plasticity in childhood brain disorders: when, what, how, and whom to assess
Abstract
At every point in the lifespan, the brain balances malleable processes representing neural plasticity that promote change with homeostatic processes that promote stability. Whether a child develops typically or with brain injury, his or her neural and behavioral outcome is constructed through transactions between plastic and homeostatic processes and the environment. In clinical research with children in whom the developing brain has been malformed or injured, behavioral outcomes provide an index of the result of plasticity, homeostasis, and environmental transactions. When should we assess outcome in relation to age at brain insult, time since brain insult, and age of the child at testing? What should we measure? Functions involving reacting to the past and predicting the future, as well as social-affective skills, are important. How should we assess outcome? Information from performance variability, direct measures and informants, overt and covert measures, and laboratory and ecological measures should be considered. In whom are we assessing outcome? Assessment should be cognizant of individual differences in gene, socio-economic status (SES), parenting, nutrition, and interpersonal supports, which are moderators that interact with other factors influencing functional outcome.
Figures
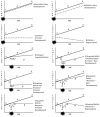
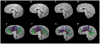
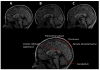
posterior lobe significantly smaller
corpus medullare not different
anterior lobe enlarged.
Cerebellar volume deficits in SBM group involves a reconfiguration involving anterior lobe enlargement and posterior lobe reduction.
References
-
- Aarsen FK, Van Dongen HR, Paquier PF, Van Mourik M, Catsman-Berrevoets CE. Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology. 2004;62(8):1311–1316. - PubMed
-
- Anderson VA, Anderson P, Northam E, Jacobs R, Mikiewicz O. Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychol. 2002;8(4):231–240. - PubMed
-
- Anderson V, Anderson D, Anderson P. Comparing attentional skills in children with acquired and developmental central nervous system disorders. J Int Neuropsychol Soc. 2006;12(4):519–531. - PubMed
-
- Ater JL, Moore BD, 3rd, Francis DJ, Castillo R, Slopis J, Copeland DR. Correlation of medical and neurosurgical events with neuropsychological status in children at diagnosis of astrocytoma: utilization of a neurological severity score. J Child Neurol. 1996;11(6):462–469. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

