Biosynthetic modularity rules in the bisintercalator family of antitumor compounds
- PMID: 24821625
- PMCID: PMC4052310
- DOI: 10.3390/md12052668
Biosynthetic modularity rules in the bisintercalator family of antitumor compounds
Abstract
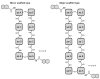
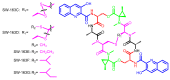
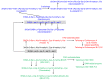
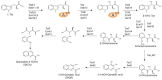
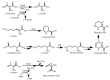
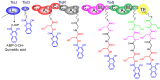
Diverse actinomycetes produce a family of structurally and biosynthetically related non-ribosomal peptide compounds which belong to the chromodepsipeptide family. These compounds act as bisintercalators into the DNA helix. They give rise to antitumor, antiparasitic, antibacterial and antiviral bioactivities. These compounds show a high degree of conserved modularity (chromophores, number and type of amino acids). This modularity and their high sequence similarities at the genetic level imply a common biosynthetic origin for these pathways. Here, we describe insights about rules governing this modular biosynthesis, taking advantage of the fact that nowadays five of these gene clusters have been made public (thiocoraline, triostin, SW-163 and echinomycin/quinomycin). This modularity has potential application for designing and producing novel genetic engineered derivatives, as well as for developing new chemical synthesis strategies. These would facilitate their clinical development.
Figures
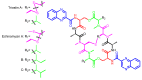
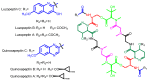
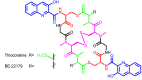
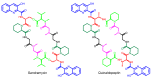

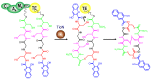
References
-
- Quigley G.J., Ughetto G., Van der Marel G.A., Van Boom J.H., Wang A.H., Rich A. Non-Watson-Crick G.C and A.T base pairs in a DNA-antibiotic complex. Science. 1986;232:1255–1258. - PubMed
-
- Park J.Y., Ryang Y.S., Shim K.Y., Lee J.I., Kim H.S., Kim Y.H., Kim S.K. Molecular signaling cascade in DNA bisintercalator, echinomycin-induced apoptosis of HT-29 cells: Evidence of the apoptotic process via activation of the cytochrome c-ERK-caspase-3 pathway. Int. J. Biochem. Cell Biol. 2006;38:244–254. doi: 10.1016/j.biocel.2005.09.003. - DOI - PubMed
-
- Corbaz R., Ettlinger L., Gäumann E., Keller-Schierlein W., Kradolfer F., Neipp L., Prelog V., Reusser P., Zähner H. Stoffwechselprodukte von Actinomyceten. 7. Echinomycin. Helv. Chim. Acta. 1957;40:199–204. doi: 10.1002/hlca.19570400124. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

