Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography
- PMID: 24821719
- PMCID: PMC4140266
- DOI: 10.1074/jbc.M114.560748
Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography
Abstract
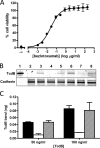
The symptoms of Clostridium difficile infections are caused by two exotoxins, TcdA and TcdB, which target host colonocytes by binding to unknown cell surface receptors, at least in part via their combined repetitive oligopeptide (CROP) domains. A combination of the anti-TcdA antibody actoxumab and the anti-TcdB antibody bezlotoxumab is currently under development for the prevention of recurrent C. difficile infections. We demonstrate here through various biophysical approaches that bezlotoxumab binds to specific regions within the N-terminal half of the TcdB CROP domain. Based on this information, we solved the x-ray structure of the N-terminal half of the TcdB CROP domain bound to Fab fragments of bezlotoxumab. The structure reveals that the TcdB CROP domain adopts a β-solenoid fold consisting of long and short repeats and that bezlotoxumab binds to two homologous sites within the CROP domain, partially occluding two of the four putative carbohydrate binding pockets located in TcdB. We also show that bezlotoxumab neutralizes TcdB by blocking binding of TcdB to mammalian cells. Overall, our data are consistent with a model wherein a single molecule of bezlotoxumab neutralizes TcdB by binding via its two Fab regions to two epitopes within the N-terminal half of the TcdB CROP domain, partially blocking the carbohydrate binding pockets of the toxin and preventing toxin binding to host cells.
Keywords: Antibody; Bacterial Toxin; Crystal Structure; Drug Action; Epitope Mapping.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







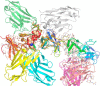
Similar articles
-
Epitopes and Mechanism of Action of the Clostridium difficile Toxin A-Neutralizing Antibody Actoxumab.J Mol Biol. 2017 Apr 7;429(7):1030-1044. doi: 10.1016/j.jmb.2017.02.010. Epub 2017 Feb 21. J Mol Biol. 2017. PMID: 28232034 Free PMC article.
-
Structural basis for antibody recognition in the receptor-binding domains of toxins A and B from Clostridium difficile.J Biol Chem. 2014 Jan 24;289(4):2331-43. doi: 10.1074/jbc.M113.505917. Epub 2013 Dec 5. J Biol Chem. 2014. PMID: 24311789 Free PMC article.
-
Use of a neutralizing antibody helps identify structural features critical for binding of Clostridium difficile toxin TcdA to the host cell surface.J Biol Chem. 2017 Sep 1;292(35):14401-14412. doi: 10.1074/jbc.M117.781112. Epub 2017 Jul 13. J Biol Chem. 2017. PMID: 28705932 Free PMC article.
-
Actoxumab + bezlotoxumab combination: what promise for Clostridium difficile treatment?Expert Opin Biol Ther. 2018 Apr;18(4):469-476. doi: 10.1080/14712598.2018.1452908. Epub 2018 Mar 15. Expert Opin Biol Ther. 2018. PMID: 29534621 Review.
-
Toxin-specific antibodies for the treatment of Clostridium difficile: current status and future perspectives.Toxins (Basel). 2010 May;2(5):998-1018. doi: 10.3390/toxins2050998. Epub 2010 May 7. Toxins (Basel). 2010. PMID: 22069622 Free PMC article. Review.
Cited by
-
Inhibition of Pore-Forming Proteins.Toxins (Basel). 2019 Sep 19;11(9):545. doi: 10.3390/toxins11090545. Toxins (Basel). 2019. PMID: 31546810 Free PMC article. Review.
-
Clostridioides difficile toxins: mechanisms of action and antitoxin therapeutics.Nat Rev Microbiol. 2022 May;20(5):285-298. doi: 10.1038/s41579-021-00660-2. Epub 2021 Nov 26. Nat Rev Microbiol. 2022. PMID: 34837014 Free PMC article. Review.
-
Whole-organism phenotypic screening for anti-infectives promoting host health.Nat Chem Biol. 2018 Apr;14(4):331-341. doi: 10.1038/s41589-018-0018-3. Epub 2018 Mar 19. Nat Chem Biol. 2018. PMID: 29556098 Free PMC article. Review.
-
Bezlotoxumab.Hosp Pharm. 2017 Mar;52(3):229-233. doi: 10.1310/hpj5203-229. Hosp Pharm. 2017. PMID: 28439138 Free PMC article.
-
Bezlotoxumab for Preventing Recurrent Clostridioides difficile Infection: A Narrative Review from Pathophysiology to Clinical Studies.Infect Dis Ther. 2020 Sep;9(3):481-494. doi: 10.1007/s40121-020-00314-5. Epub 2020 Jul 6. Infect Dis Ther. 2020. PMID: 32632582 Free PMC article. Review.
References
-
- Rupnik M., Wilcox M. H., Gerding D. N. (2009) Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7, 526–536 - PubMed
-
- Bassetti M., Villa G., Pecori D., Arzese A., Wilcox M. (2012) Epidemiology, diagnosis and treatment of Clostridium difficile infection. Expert Rev. Anti. Infect. Ther. 10, 1405–1423 - PubMed
-
- Carter G. P., Rood J. I., Lyras D. (2012) The role of toxin A and toxin B in the virulence of Clostridium difficile. Trends Microbiol. 20, 21–29 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

