Launching PCORnet, a national patient-centered clinical research network
- PMID: 24821743
- PMCID: PMC4078292
- DOI: 10.1136/amiajnl-2014-002747
Launching PCORnet, a national patient-centered clinical research network
Abstract
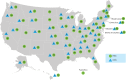
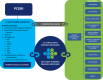
The Patient-Centered Outcomes Research Institute (PCORI) has launched PCORnet, a major initiative to support an effective, sustainable national research infrastructure that will advance the use of electronic health data in comparative effectiveness research (CER) and other types of research. In December 2013, PCORI's board of governors funded 11 clinical data research networks (CDRNs) and 18 patient-powered research networks (PPRNs) for a period of 18 months. CDRNs are based on the electronic health records and other electronic sources of very large populations receiving healthcare within integrated or networked delivery systems. PPRNs are built primarily by communities of motivated patients, forming partnerships with researchers. These patients intend to participate in clinical research, by generating questions, sharing data, volunteering for interventional trials, and interpreting and disseminating results. Rapidly building a new national resource to facilitate a large-scale, patient-centered CER is associated with a number of technical, regulatory, and organizational challenges, which are described here.
Keywords: clinical data research networks; comparative effectiveness research; distributed databases; patient-centered outcomes research institute; patient-powered research networks.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- Institute of Medicine (U.S.). Committee on comparative effectiveness research prioritization. Initial national priorities for comparative effectiveness research. Washington, DC: National Academies Press, 2009
-
- PCORI. National workshop report: advancing the use of electronic data in patient-centered outcomes research. Washington, DC: PCORI, 2013
-
- Vogt TM, Elston-Lafata J, Tolsma D, et al. The role of research in integrated healthcare systems: the HMO Research Network. Am J Manag Care 2004;10:643–8 - PubMed
-
- EDM Forum. Building the Electronic Clinical Data Infrastructure to Improve Patient Outcomes: CER Project Profiles. Issue Briefs and Reports: Academy Health, 2012
-
- Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127(Suppl 1):S45–53 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

