Split-gene system for hybrid wheat seed production
- PMID: 24821800
- PMCID: PMC4078799
- DOI: 10.1073/pnas.1402836111
Split-gene system for hybrid wheat seed production
Abstract
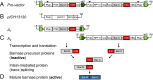
Hybrid wheat plants are superior in yield and growth characteristics compared with their homozygous parents. The commercial production of wheat hybrids is difficult because of the inbreeding nature of wheat and the lack of a practical fertility control that enforces outcrossing. We describe a hybrid wheat system that relies on the expression of a phytotoxic barnase and provides for male sterility. The barnase coding information is divided and distributed at two loci that are located on allelic positions of the host chromosome and are therefore "linked in repulsion." Functional complementation of the loci is achieved through coexpression of the barnase fragments and intein-mediated ligation of the barnase protein fragments. This system allows for growth and maintenance of male-sterile female crossing partners, whereas the hybrids are fertile. The technology does not require fertility restorers and is based solely on the genetic modification of the female crossing partner.
Keywords: hybrid wheat breeding; intein-mediated protein splicing; site-specific recombination; split-gene approach.
Conflict of interest statement
Conflict of interest statement: The cooperation partner Nordsaat Saatzucht GmbH holds a patent that protects key elements of the split-gene system (Eur Patent Publ EP2294204).
Figures


Comment in
-
Rewiring the wheat reproductive system to harness heterosis for the next wave of yield improvement.Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):9024-5. doi: 10.1073/pnas.1407956111. Epub 2014 Jun 16. Proc Natl Acad Sci U S A. 2014. PMID: 24963159 Free PMC article. No abstract available.
References
-
- Schnable PS, Springer NM. Progress toward understanding heterosis in crop plants. Annu Rev Plant Biol. 2013;64:71–88. - PubMed
-
- Kempe K, Gils M. Pollination control technologies for hybrid breeding. Plant Breed. 2011;27(4):417–437.
-
- Perez-Prat E, van Lookeren Campagne MM. Hybrid seed production and the challenge of propagating male-sterile plants. Trends Plant Sci. 2002;7(5):199–203. - PubMed
-
- Smith CW, Betrán J, Runge E. Corn: Origin, History, Technology, and Production. Hoboken, NJ: Wiley; 2004.
-
- Chen L, Liu Y-G. Male sterility and fertility restoration in crops. Annu Rev Plant Biol. 2014;65:5.1–5.28. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

