Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity
- PMID: 24821881
- PMCID: PMC4050203
- DOI: 10.1200/JCO.2013.53.2465
Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity
Abstract
Purpose: Neoadjuvant cisplatin-based chemotherapy is standard of care for muscle-invasive bladder cancer (MIBC); however, it is infrequently adopted in practice because of concerns regarding toxicity and delay to cystectomy. We hypothesized that three cycles of neoadjuvant accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (AMVAC) would be safe, shorten the time to surgery, and yield similar pathologic complete response (pT0) rates compared with historical controls.
Patients and methods: Patients with cT2-T4a and N0-N1 MIBC were eligible and received three cycles of AMVAC with pegfilgrastim followed by radical cystectomy with lymph node dissection. The primary end point was pT0 rate. Telomere length (TL) and p53 mutation status were correlated with response and toxicity.
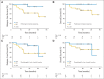
Results: Forty-four patients were accrued; 60% had stage III to IV disease; median age was 64 years. Forty patients were evaluable for response, with 15 (38%; 95% CI, 23% to 53%) showing pT0 at cystectomy, meeting the primary end point of the study. Another six patients (14%) were downstaged to non-muscle invasive disease. Most (82%) experienced only grade 1 to 2 treatment-related toxicities. There were no grade 3 or 4 renal toxicities and no treatment-related deaths. One patient developed metastases and thus did not undergo cystectomy; all others (n = 43) proceeded to cystectomy within 8 weeks after last chemotherapy administration. Median time from start of chemotherapy to cystectomy was 9.7 weeks. TL and p53 mutation did not predict response or toxicity.
Conclusion: AMVAC is well tolerated and results in similar pT0 rates with 6 weeks of treatment compared with standard 12-week regimens. Further analysis is ongoing to ascertain whether molecular alterations in tumor samples can predict response to chemotherapy.
Trial registration: ClinicalTrials.gov NCT01031420.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Everything old is new again! Neoadjuvant chemotherapy in the treatment of muscle-invasive bladder cancer.J Clin Oncol. 2014 Jun 20;32(18):1868-70. doi: 10.1200/JCO.2014.55.4055. Epub 2014 May 12. J Clin Oncol. 2014. PMID: 24821880 No abstract available.
-
Bladder cancer: Accelerating MVAC.Nat Rev Urol. 2014 Jun;11(6):307. doi: 10.1038/nrurol.2014.128. Epub 2014 May 27. Nat Rev Urol. 2014. PMID: 24861327 No abstract available.
-
Bladder cancer: accelerating MVAC.Nat Rev Clin Oncol. 2014 Jul;11(7):378. doi: 10.1038/nrclinonc.2014.94. Epub 2014 May 27. Nat Rev Clin Oncol. 2014. PMID: 24863165 No abstract available.
-
Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy in bladder cancer: ready for prime time?J Clin Oncol. 2014 Dec 20;32(36):4168-9. doi: 10.1200/JCO.2014.58.0498. Epub 2014 Nov 10. J Clin Oncol. 2014. PMID: 25385728 No abstract available.
-
Reply to D. Pouessel et al, J.B. Aragon-Ching, and B.A. Adesunloye.J Clin Oncol. 2014 Dec 20;32(36):4171-2. doi: 10.1200/JCO.2014.58.5281. Epub 2014 Nov 10. J Clin Oncol. 2014. PMID: 25385730 No abstract available.
-
Neoadjuvant phase II studies of modified methotrexate, vinblastine, doxorubicin, and cisplatin in muscle-invasive bladder cancer.J Clin Oncol. 2014 Dec 20;32(36):4170. doi: 10.1200/JCO.2014.57.9318. Epub 2014 Nov 10. J Clin Oncol. 2014. PMID: 25385733 No abstract available.
-
Neoadjuvant chemotherapy for muscle-invasive bladder cancer: are we asking the right questions?J Clin Oncol. 2014 Dec 20;32(36):4169-70. doi: 10.1200/JCO.2014.57.3154. Epub 2014 Nov 10. J Clin Oncol. 2014. PMID: 25385734 No abstract available.
-
Re: Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity.J Urol. 2015 Jun;193(6):1932. doi: 10.1016/j.juro.2015.03.011. Epub 2015 Mar 13. J Urol. 2015. PMID: 25986790 No abstract available.
References
-
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. - PubMed
-
- Neoadjuvant chemotherapy in invasive bladder cancer. Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205. discussion 205-206. - PubMed
-
- Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer. A randomised controlled trial—International collaboration of trialists. Lancet. 1999;354:533–540. - PubMed
-
- Montie JE, Clark PE, Eisenberger MA, et al. Bladder cancer. J Natl Compr Canc Netw. 2009;7:8–39. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous