Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates
- PMID: 24821883
- PMCID: PMC7057274
- DOI: 10.1200/JCO.2013.52.4785
Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates
Abstract
Purpose: In advanced urothelial cancer, treatment with dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) results in a high response rate, less toxicity, and few dosing delays. We explored the efficacy and safety of neoadjuvant ddMVAC with pegfilgrastim support in muscle-invasive urothelial cancer (MIUC).
Patients and methods: Patients with cT2-cT4, N0-1, M0 MIUC were enrolled. Four cycles of ddMVAC were administered, followed by radical cystectomy. The primary end point was pathologic response (PaR) defined by pathologic downstaging to ≤ pT1N0M0. The study used Simon's optimal two-stage design to evaluate null and alternative hypotheses of PaR rate of 35% versus 55%. Secondary end points included toxicity, disease-free survival (DFS), radiologic response (RaR), and biomarker correlates, including ERCC1.
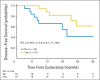
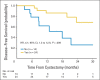
Results: Between December 2008 and April 2012, 39 patients (cT2N0, 33%; cT3N0, 18%; cT4N0, 3%; cT2-4N1, 43%; unspecified, 3%) were enrolled. Median follow-up was 2 years. Overall, 49% (80% CI, 38 to 61) achieved PaR of ≤ pT1N0M0, and we concluded this regimen was effective. High-grade (grade ≥ 3) toxicities were observed in 10% of patients, with no neutropenic fevers or treatment-related death. One-year DFS was 89% versus 67% for patients who achieved PaR compared with those who did not (hazard ratio [HR], 2.6; 95% CI, 0.8 to 8.1; P = .08) and 86% versus 62% for patients who achieved RaR compared with those who did not (HR, 4.1; 95% CI, 1.3 to 12.5; P = .009). We found no association between serum tumor markers or ERCC1 expression with response or survival.
Conclusion: In patients with MIUC, neoadjuvant ddMVAC was well tolerated and resulted in significant pathologic and radiologic downstaging.
Trial registration: ClinicalTrials.gov NCT00808639.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Everything old is new again! Neoadjuvant chemotherapy in the treatment of muscle-invasive bladder cancer.J Clin Oncol. 2014 Jun 20;32(18):1868-70. doi: 10.1200/JCO.2014.55.4055. Epub 2014 May 12. J Clin Oncol. 2014. PMID: 24821880 No abstract available.
-
Bladder cancer: Accelerating MVAC.Nat Rev Urol. 2014 Jun;11(6):307. doi: 10.1038/nrurol.2014.128. Epub 2014 May 27. Nat Rev Urol. 2014. PMID: 24861327 No abstract available.
-
Bladder cancer: accelerating MVAC.Nat Rev Clin Oncol. 2014 Jul;11(7):378. doi: 10.1038/nrclinonc.2014.94. Epub 2014 May 27. Nat Rev Clin Oncol. 2014. PMID: 24863165 No abstract available.
-
Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy in bladder cancer: ready for prime time?J Clin Oncol. 2014 Dec 20;32(36):4168-9. doi: 10.1200/JCO.2014.58.0498. Epub 2014 Nov 10. J Clin Oncol. 2014. PMID: 25385728 No abstract available.
-
Neoadjuvant phase II studies of modified methotrexate, vinblastine, doxorubicin, and cisplatin in muscle-invasive bladder cancer.J Clin Oncol. 2014 Dec 20;32(36):4170. doi: 10.1200/JCO.2014.57.9318. Epub 2014 Nov 10. J Clin Oncol. 2014. PMID: 25385733 No abstract available.
-
Neoadjuvant chemotherapy for muscle-invasive bladder cancer: are we asking the right questions?J Clin Oncol. 2014 Dec 20;32(36):4169-70. doi: 10.1200/JCO.2014.57.3154. Epub 2014 Nov 10. J Clin Oncol. 2014. PMID: 25385734 No abstract available.
References
-
- Grossman HB Natale RB Tangen CM , etal: Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer N Engl J Med 349: 859– 866,2003. - PubMed
-
- Sternberg CN Yagoda A Scher HI , etal: Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium: Efficacy and patterns of response and relapse Cancer 64: 2448– 2458,1989. - PubMed
-
- Loehrer PJ Sr Einhorn LH Elson PJ , etal: A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study J Clin Oncol 10: 1066– 1073,1992. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

