A novel Aβ-fibrinogen interaction inhibitor rescues altered thrombosis and cognitive decline in Alzheimer's disease mice
- PMID: 24821909
- PMCID: PMC4042638
- DOI: 10.1084/jem.20131751
A novel Aβ-fibrinogen interaction inhibitor rescues altered thrombosis and cognitive decline in Alzheimer's disease mice
Abstract
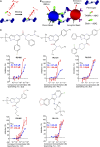
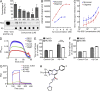
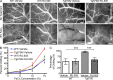
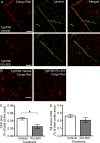
Many Alzheimer's disease (AD) patients suffer from cerebrovascular abnormalities such as altered cerebral blood flow and cerebral microinfarcts. Recently, fibrinogen has been identified as a strong cerebrovascular risk factor in AD, as it specifically binds to β-amyloid (Aβ), thereby altering fibrin clot structure and delaying clot degradation. To determine if the Aβ-fibrinogen interaction could be targeted as a potential new treatment for AD, we designed a high-throughput screen and identified RU-505 as an effective inhibitor of the Aβ-fibrinogen interaction. RU-505 restored Aβ-induced altered fibrin clot formation and degradation in vitro and inhibited vessel occlusion in AD transgenic mice. Furthermore, long-term treatment of RU-505 significantly reduced vascular amyloid deposition and microgliosis in the cortex and improved cognitive impairment in mouse models of AD. Our studies suggest that inhibitors targeting the Aβ-fibrinogen interaction show promise as therapy for treating AD.
© 2014 Ahn et al.
Figures


Comment in
-
Alzheimer's disease: Aβ-fibrinogen interaction inhibitor improves cognition in AD.Nat Rev Drug Discov. 2014 Jul;13(7):494. doi: 10.1038/nrd4370. Epub 2014 Jun 20. Nat Rev Drug Discov. 2014. PMID: 24948119 No abstract available.
References
-
- Adams R.A., Bauer J., Flick M.J., Sikorski S.L., Nuriel T., Lassmann H., Degen J.L., Akassoglou K. 2007. The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J. Exp. Med. 204:571–582 10.1084/jem.20061931 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

