Ion channel regulation by protein S-acylation
- PMID: 24821965
- PMCID: PMC4035745
- DOI: 10.1085/jgp.201411176
Ion channel regulation by protein S-acylation
Abstract
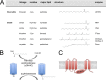
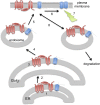
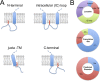
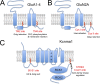
Protein S-acylation, the reversible covalent fatty-acid modification of cysteine residues, has emerged as a dynamic posttranslational modification (PTM) that controls the diversity, life cycle, and physiological function of numerous ligand- and voltage-gated ion channels. S-acylation is enzymatically mediated by a diverse family of acyltransferases (zDHHCs) and is reversed by acylthioesterases. However, for most ion channels, the dynamics and subcellular localization at which S-acylation and deacylation cycles occur are not known. S-acylation can control the two fundamental determinants of ion channel function: (1) the number of channels resident in a membrane and (2) the activity of the channel at the membrane. It controls the former by regulating channel trafficking and the latter by controlling channel kinetics and modulation by other PTMs. Ion channel function may be modulated by S-acylation of both pore-forming and regulatory subunits as well as through control of adapter, signaling, and scaffolding proteins in ion channel complexes. Importantly, cross-talk of S-acylation with other PTMs of both cysteine residues by themselves and neighboring sites of phosphorylation is an emerging concept in the control of ion channel physiology. In this review, I discuss the fundamentals of protein S-acylation and the tools available to investigate ion channel S-acylation. The mechanisms and role of S-acylation in controlling diverse stages of the ion channel life cycle and its effect on ion channel function are highlighted. Finally, I discuss future goals and challenges for the field to understand both the mechanistic basis for S-acylation control of ion channels and the functional consequence and implications for understanding the physiological function of ion channel S-acylation in health and disease.
© 2014 Shipston.
Figures

Similar articles
-
Palmitoylation of Voltage-Gated Ion Channels.Int J Mol Sci. 2022 Aug 19;23(16):9357. doi: 10.3390/ijms23169357. Int J Mol Sci. 2022. PMID: 36012639 Free PMC article. Review.
-
S-acylation dependent post-translational cross-talk regulates large conductance calcium- and voltage- activated potassium (BK) channels.Front Physiol. 2014 Aug 5;5:281. doi: 10.3389/fphys.2014.00281. eCollection 2014. Front Physiol. 2014. PMID: 25140154 Free PMC article. Review.
-
Ion channel regulation by protein palmitoylation.J Biol Chem. 2011 Mar 18;286(11):8709-16. doi: 10.1074/jbc.R110.210005. Epub 2011 Jan 7. J Biol Chem. 2011. PMID: 21216969 Free PMC article. Review.
-
S-acylation regulates Kv1.5 channel surface expression.Am J Physiol Cell Physiol. 2007 Jul;293(1):C152-61. doi: 10.1152/ajpcell.00480.2006. Epub 2007 Mar 7. Am J Physiol Cell Physiol. 2007. PMID: 17344312
-
S-Acylation controls functional coupling of BK channel pore-forming α-subunits and β1-subunits.J Biol Chem. 2019 Aug 9;294(32):12066-12076. doi: 10.1074/jbc.RA119.009065. Epub 2019 Jun 18. J Biol Chem. 2019. PMID: 31213527 Free PMC article.
Cited by
-
Palmitoylation: A Fatty Regulator of Myocardial Electrophysiology.Front Physiol. 2020 Feb 19;11:108. doi: 10.3389/fphys.2020.00108. eCollection 2020. Front Physiol. 2020. PMID: 32140110 Free PMC article. Review.
-
The direct modulatory activity of zinc toward ion channels.Integr Med Res. 2015 Sep;4(3):142-146. doi: 10.1016/j.imr.2015.07.004. Epub 2015 Jul 15. Integr Med Res. 2015. PMID: 28664120 Free PMC article. Review.
-
Palmitoylation of Voltage-Gated Ion Channels.Int J Mol Sci. 2022 Aug 19;23(16):9357. doi: 10.3390/ijms23169357. Int J Mol Sci. 2022. PMID: 36012639 Free PMC article. Review.
-
Posttranslational Protein Modifications in Plant Metabolism.Plant Physiol. 2015 Nov;169(3):1469-87. doi: 10.1104/pp.15.01378. Epub 2015 Sep 3. Plant Physiol. 2015. PMID: 26338952 Free PMC article.
-
Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization.Cell. 2019 Oct 17;179(3):659-670.e13. doi: 10.1016/j.cell.2019.09.017. Epub 2019 Oct 3. Cell. 2019. PMID: 31587896 Free PMC article.
References
-
- Adibekian A., Martin B.R., Chang J.W., Hsu K.-L., Tsuboi K., Bachovchin D.A., Speers A.E., Brown S.J., Spicer T., Fernandez-Vega V., et al. 2012. Confirming target engagement for reversible inhibitors in vivo by kinetically tuned activity-based probes. J. Am. Chem. Soc. 134:10345–10348 10.1021/ja303400u - DOI - PMC - PubMed
-
- Amici S.A., McKay S.B., Wells G.B., Robson J.I., Nasir M., Ponath G., Anand R. 2012. A highly conserved cytoplasmic cysteine residue in the α4 nicotinic acetylcholine receptor is palmitoylated and regulates protein expression. J. Biol. Chem. 287:23119–23127 10.1074/jbc.M111.328294 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

