Ion channel regulation by protein S-acylation
- PMID: 24821965
- PMCID: PMC4035745
- DOI: 10.1085/jgp.201411176
Ion channel regulation by protein S-acylation
Abstract
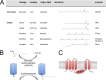
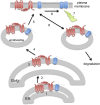
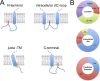
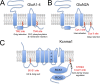
Protein S-acylation, the reversible covalent fatty-acid modification of cysteine residues, has emerged as a dynamic posttranslational modification (PTM) that controls the diversity, life cycle, and physiological function of numerous ligand- and voltage-gated ion channels. S-acylation is enzymatically mediated by a diverse family of acyltransferases (zDHHCs) and is reversed by acylthioesterases. However, for most ion channels, the dynamics and subcellular localization at which S-acylation and deacylation cycles occur are not known. S-acylation can control the two fundamental determinants of ion channel function: (1) the number of channels resident in a membrane and (2) the activity of the channel at the membrane. It controls the former by regulating channel trafficking and the latter by controlling channel kinetics and modulation by other PTMs. Ion channel function may be modulated by S-acylation of both pore-forming and regulatory subunits as well as through control of adapter, signaling, and scaffolding proteins in ion channel complexes. Importantly, cross-talk of S-acylation with other PTMs of both cysteine residues by themselves and neighboring sites of phosphorylation is an emerging concept in the control of ion channel physiology. In this review, I discuss the fundamentals of protein S-acylation and the tools available to investigate ion channel S-acylation. The mechanisms and role of S-acylation in controlling diverse stages of the ion channel life cycle and its effect on ion channel function are highlighted. Finally, I discuss future goals and challenges for the field to understand both the mechanistic basis for S-acylation control of ion channels and the functional consequence and implications for understanding the physiological function of ion channel S-acylation in health and disease.
© 2014 Shipston.
Figures

References
-
- Adibekian A., Martin B.R., Chang J.W., Hsu K.-L., Tsuboi K., Bachovchin D.A., Speers A.E., Brown S.J., Spicer T., Fernandez-Vega V., et al. 2012. Confirming target engagement for reversible inhibitors in vivo by kinetically tuned activity-based probes. J. Am. Chem. Soc. 134:10345–10348 10.1021/ja303400u - DOI - PMC - PubMed
-
- Amici S.A., McKay S.B., Wells G.B., Robson J.I., Nasir M., Ponath G., Anand R. 2012. A highly conserved cytoplasmic cysteine residue in the α4 nicotinic acetylcholine receptor is palmitoylated and regulates protein expression. J. Biol. Chem. 287:23119–23127 10.1074/jbc.M111.328294 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

