Coincidence detection in the medial superior olive: mechanistic implications of an analysis of input spiking patterns
- PMID: 24822037
- PMCID: PMC4013490
- DOI: 10.3389/fncir.2014.00042
Coincidence detection in the medial superior olive: mechanistic implications of an analysis of input spiking patterns
Abstract
Coincidence detection by binaural neurons in the medial superior olive underlies sensitivity to interaural time difference (ITD) and interaural correlation (ρ). It is unclear whether this process is akin to a counting of individual coinciding spikes, or rather to a correlation of membrane potential waveforms resulting from converging inputs from each side. We analyzed spike trains of axons of the cat trapezoid body (TB) and auditory nerve (AN) in a binaural coincidence scheme. ITD was studied by delaying "ipsi-" vs. "contralateral" inputs; ρ was studied by using responses to different noises. We varied the number of inputs; the monaural and binaural threshold and the coincidence window duration. We examined physiological plausibility of output "spike trains" by comparing their rate and tuning to ITD and ρ to those of binaural cells. We found that multiple inputs are required to obtain a plausible output spike rate. In contrast to previous suggestions, monaural threshold almost invariably needed to exceed binaural threshold. Elevation of the binaural threshold to values larger than 2 spikes caused a drastic decrease in rate for a short coincidence window. Longer coincidence windows allowed a lower number of inputs and higher binaural thresholds, but decreased the depth of modulation. Compared to AN fibers, TB fibers allowed higher output spike rates for a low number of inputs, but also generated more monaural coincidences. We conclude that, within the parameter space explored, the temporal patterns of monaural fibers require convergence of multiple inputs to achieve physiological binaural spike rates; that monaural coincidences have to be suppressed relative to binaural ones; and that the neuron has to be sensitive to single binaural coincidences of spikes, for a number of excitatory inputs per side of 10 or less. These findings suggest that the fundamental operation in the mammalian binaural circuit is coincidence counting of single binaural input spikes.
Keywords: auditory nerve; coincidence detection; coincidence window; input convergence; interaural correlation; interaural time difference; medial superior olive; temporal coding.
Figures
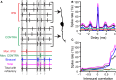

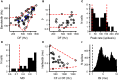
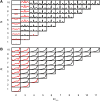
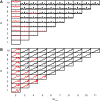







Similar articles
-
Roles for Coincidence Detection in Coding Amplitude-Modulated Sounds.PLoS Comput Biol. 2016 Jun 20;12(6):e1004997. doi: 10.1371/journal.pcbi.1004997. eCollection 2016 Jun. PLoS Comput Biol. 2016. PMID: 27322612 Free PMC article.
-
On the precision of neural computation with interaural level differences in the lateral superior olive.Brain Res. 2013 Nov 6;1536:16-26. doi: 10.1016/j.brainres.2013.05.008. Epub 2013 May 14. Brain Res. 2013. PMID: 23684714
-
Envelope coding in the lateral superior olive. II. Characteristic delays and comparison with responses in the medial superior olive.J Neurophysiol. 1996 Oct;76(4):2137-56. doi: 10.1152/jn.1996.76.4.2137. J Neurophysiol. 1996. PMID: 8899590
-
The volley theory and the spherical cell puzzle.Neuroscience. 2008 Jun 12;154(1):65-76. doi: 10.1016/j.neuroscience.2008.03.002. Epub 2008 Mar 8. Neuroscience. 2008. PMID: 18424004 Free PMC article. Review.
-
The function of the medial superior olive in small mammals: temporal receptive fields in auditory analysis.J Comp Physiol A. 2000 May;186(5):413-23. doi: 10.1007/s003590050441. J Comp Physiol A. 2000. PMID: 10879945 Review.
Cited by
-
In vivo coincidence detection in mammalian sound localization generates phase delays.Nat Neurosci. 2015 Mar;18(3):444-52. doi: 10.1038/nn.3948. Epub 2015 Feb 9. Nat Neurosci. 2015. PMID: 25664914 Free PMC article.
-
Predicting binaural responses from monaural responses in the gerbil medial superior olive.J Neurophysiol. 2016 Jun 1;115(6):2950-63. doi: 10.1152/jn.01146.2015. Epub 2016 Mar 23. J Neurophysiol. 2016. PMID: 27009164 Free PMC article.
-
Auditory midbrain representation of a break in interaural correlation.J Neurophysiol. 2015 Oct;114(4):2258-64. doi: 10.1152/jn.00645.2015. Epub 2015 Aug 12. J Neurophysiol. 2015. PMID: 26269559 Free PMC article.
-
Cortical mechanisms of spatial hearing.Nat Rev Neurosci. 2019 Oct;20(10):609-623. doi: 10.1038/s41583-019-0206-5. Epub 2019 Aug 29. Nat Rev Neurosci. 2019. PMID: 31467450 Free PMC article. Review.
-
Physiological models of the lateral superior olive.PLoS Comput Biol. 2017 Dec 27;13(12):e1005903. doi: 10.1371/journal.pcbi.1005903. eCollection 2017 Dec. PLoS Comput Biol. 2017. PMID: 29281618 Free PMC article.
References
-
- Albeck Y., Konishi M. (1995). Reponses of neurons in the auditory pathway of the barn owl to partially correlated binaural signals. J. Neurophysiol. 74, 1689–1700 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

