Plasmodium falciparum induces Foxp3hi CD4 T cells independent of surface PfEMP1 expression via small soluble parasite components
- PMID: 24822053
- PMCID: PMC4013457
- DOI: 10.3389/fmicb.2014.00200
Plasmodium falciparum induces Foxp3hi CD4 T cells independent of surface PfEMP1 expression via small soluble parasite components
Abstract
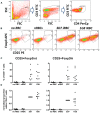
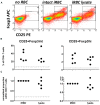
Elevated levels of regulatory T cells following Plasmodium infection are a well-reported phenomenon that can influence both protective and pathological anti-parasite responses, and might additionally impact on vaccine responses in acutely malaria infected individuals. The mechanisms underlying their induction or expansion by the parasite, however, are incompletely understood. In a previous study, Plasmodium falciparum infected red blood cells (iRBCs) were shown to induce effector-cytokine producing Foxp3int CD4+ T cells, as well as regulatory Foxp3hi CD4+ T cells in vitro. The aim of the present study was to determine the contribution of parasite components to the induction of Foxp3 expression in human CD4+ T cells. Using the surface PfEMP1-deficient parasite line 1G8, we demonstrate that induction of Foxp3hi and Foxp3int CD4+ T cells is independent of PfEMP1 expression on iRBCs. We further demonstrate that integrity of iRBCs is no requirement for the induction of Foxp3 expression. Finally, transwell experiments showed that induction of Foxp3 expression, and specifically the generation of Foxp3hi as opposed to Foxp3int CD4 T cells, can be mediated by soluble parasite components smaller than 20 nm and thus likely distinct from the malaria pigment hemozoin. These results suggest that the induction of Foxp3hi T cells by P. falciparum is largely independent of two key immune modulatory parasite components, and warrant future studies into the nature of the Foxp3hi inducing parasite components to potentially allow their exclusion from vaccine formulations.
Keywords: Foxp3; Malaria; PfEMP-1; Plasmodium falciparum; hemozoin; regulatory T cell.
Figures

Similar articles
-
Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta.PLoS Pathog. 2009 Aug;5(8):e1000543. doi: 10.1371/journal.ppat.1000543. Epub 2009 Aug 14. PLoS Pathog. 2009. PMID: 19680449 Free PMC article.
-
Detection of CD4+CD45RO+ T lymphocytes producing IL-4 in response to antigens on Plasmodium falciparum erythrocytes: an in vitro correlate of protective immunity induced with attenuated Plasmodium falciparum sporozoites.Cell Immunol. 1997 Sep 15;180(2):143-52. doi: 10.1006/cimm.1997.1186. Cell Immunol. 1997. PMID: 9341744
-
Exposure-dependent control of malaria-induced inflammation in children.PLoS Pathog. 2014 Apr 17;10(4):e1004079. doi: 10.1371/journal.ppat.1004079. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24743880 Free PMC article. Clinical Trial.
-
PfEMP1 - A Parasite Protein Family of Key Importance in Plasmodium falciparum Malaria Immunity and Pathogenesis.Adv Parasitol. 2015 Apr;88:51-84. doi: 10.1016/bs.apar.2015.02.004. Epub 2015 Mar 23. Adv Parasitol. 2015. PMID: 25911365 Review.
-
Selected problems of malaria blood stage immunity.Tokai J Exp Clin Med. 1998 Apr;23(2):55-62. Tokai J Exp Clin Med. 1998. PMID: 10021776 Review.
Cited by
-
Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria.PLoS Pathog. 2015 Jul 16;11(7):e1005041. doi: 10.1371/journal.ppat.1005041. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26182204 Free PMC article.
-
Impact of exposure to malaria and nutritional status on responses to the experimental malaria vaccine ChAd63 MVA ME-TRAP in 5-17 month-old children in Burkina Faso.Front Immunol. 2022 Dec 2;13:1058227. doi: 10.3389/fimmu.2022.1058227. eCollection 2022. Front Immunol. 2022. PMID: 36532031 Free PMC article. Clinical Trial.
-
Editorial: Why Vaccines to HIV, HCV, and Malaria Have So Far Failed-Challenges to Developing Vaccines Against Immunoregulating Pathogens.Front Microbiol. 2015 Nov 27;6:1318. doi: 10.3389/fmicb.2015.01318. eCollection 2015. Front Microbiol. 2015. PMID: 26640461 Free PMC article. No abstract available.
-
Immunosuppression in Malaria: Do Plasmodium falciparum Parasites Hijack the Host?Pathogens. 2021 Oct 3;10(10):1277. doi: 10.3390/pathogens10101277. Pathogens. 2021. PMID: 34684226 Free PMC article. Review.
-
Genetics of Malaria Inflammatory Responses: A Pathogenesis Perspective.Front Immunol. 2019 Jul 30;10:1771. doi: 10.3389/fimmu.2019.01771. eCollection 2019. Front Immunol. 2019. PMID: 31417551 Free PMC article. Review.
References
-
- Brustoski K., Moller U., Kramer M., Hartgers F. C., Kremsner P. G., Krzych U., et al. (2006). Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J. Infect. Dis. 193 146–154 10.1086/498578 - DOI - PubMed
-
- Clemente A., Caporale R., Sannella A. R., Majori G., Severini C., Fadigati G., et al. (2011). Plasmodium falciparum soluble extracts potentiate the suppressive function of polyclonal T regulatory cells through activation of TGFbeta-mediated signals. Cell. Microbiol. 13 1328–1338 10.1111/j.1462-5822.2011.01622.x - DOI - PubMed
-
- Clemente A. M., Fadigati G., Caporale R., Marchese D. G., Castronovo G., Sannella A. R., et al. (2013). Modulation of the immune and inflammatory responses by Plasmodium falciparum schizont extracts: role of myeloid dendritic cells in effector and regulatory functions of CD4+ lymphocytes. Infect. Immun. 81 1842–1851 10.1128/IAI.01226-12 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

