The cyclic-di-GMP signaling pathway in the Lyme disease spirochete, Borrelia burgdorferi
- PMID: 24822172
- PMCID: PMC4013479
- DOI: 10.3389/fcimb.2014.00056
The cyclic-di-GMP signaling pathway in the Lyme disease spirochete, Borrelia burgdorferi
Abstract
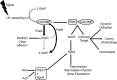
In nature, the Lyme disease spirochete Borrelia burgdorferi cycles between the unrelated environments of the Ixodes tick vector and mammalian host. In order to survive transmission between hosts, B. burgdorferi must be able to not only detect changes in its environment, but also rapidly and appropriately respond to these changes. One manner in which this obligate parasite regulates and adapts to its changing environment is through cyclic-di-GMP (c-di-GMP) signaling. c-di-GMP has been shown to be instrumental in orchestrating the adaptation of B. burgdorferi to the tick environment. B. burgdorferi possesses only one set of c-di-GMP-metabolizing genes (one diguanylate cyclase and two distinct phosphodiesterases) and one c-di-GMP-binding PilZ-domain protein designated as PlzA. While studies in the realm of c-di-GMP signaling in B. burgdorferi have exploded in the last few years, there are still many more questions than answers. Elucidation of the importance of c-di-GMP signaling to B. burgdorferi may lead to the identification of mechanisms that are critical for the survival of B. burgdorferi in the tick phase of the enzootic cycle as well as potentially delineate a role (if any) c-di-GMP may play in the transmission and virulence of B. burgdorferi during the enzootic cycle, thereby enabling the development of effective drugs for the prevention and/or treatment of Lyme disease.
Keywords: Borrelia burgdorferi; Lyme disease; c-di-GMP; chemotaxis; motility; virulence.
Figures
Similar articles
-
The Borrelia burgdorferi c-di-GMP Binding Receptors, PlzA and PlzB, Are Functionally Distinct.Front Cell Infect Microbiol. 2018 Jul 11;8:213. doi: 10.3389/fcimb.2018.00213. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30050868 Free PMC article.
-
Positive and Negative Regulation of Glycerol Utilization by the c-di-GMP Binding Protein PlzA in Borrelia burgdorferi.J Bacteriol. 2018 Oct 23;200(22):e00243-18. doi: 10.1128/JB.00243-18. Print 2018 Nov 15. J Bacteriol. 2018. PMID: 30181123 Free PMC article.
-
Analysis of the HD-GYP domain cyclic dimeric GMP phosphodiesterase reveals a role in motility and the enzootic life cycle of Borrelia burgdorferi.Infect Immun. 2011 Aug;79(8):3273-83. doi: 10.1128/IAI.05153-11. Epub 2011 Jun 13. Infect Immun. 2011. PMID: 21670168 Free PMC article.
-
Interaction of the Lyme disease spirochete with its tick vector.Cell Microbiol. 2016 Jul;18(7):919-27. doi: 10.1111/cmi.12609. Epub 2016 May 24. Cell Microbiol. 2016. PMID: 27147446 Free PMC article. Review.
-
Gene regulation in Borrelia burgdorferi.Annu Rev Microbiol. 2011;65:479-99. doi: 10.1146/annurev.micro.112408.134040. Annu Rev Microbiol. 2011. PMID: 21801026 Review.
Cited by
-
The Borrelia burgdorferi c-di-GMP Binding Receptors, PlzA and PlzB, Are Functionally Distinct.Front Cell Infect Microbiol. 2018 Jul 11;8:213. doi: 10.3389/fcimb.2018.00213. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30050868 Free PMC article.
-
The Borrelia burgdorferi Adenylate Cyclase, CyaB, Is Important for Virulence Factor Production and Mammalian Infection.Front Microbiol. 2021 May 25;12:676192. doi: 10.3389/fmicb.2021.676192. eCollection 2021. Front Microbiol. 2021. PMID: 34113333 Free PMC article.
-
The Borrelia burgdorferi CheY3 response regulator is essential for chemotaxis and completion of its natural infection cycle.Cell Microbiol. 2016 Dec;18(12):1782-1799. doi: 10.1111/cmi.12617. Epub 2016 Jul 11. Cell Microbiol. 2016. PMID: 27206578 Free PMC article.
-
LtpA, a CdnL-type CarD regulator, is important for the enzootic cycle of the Lyme disease pathogen.Emerg Microbes Infect. 2018 Jul 9;7(1):126. doi: 10.1038/s41426-018-0122-1. Emerg Microbes Infect. 2018. PMID: 29985409 Free PMC article.
-
Gene Regulation and Transcriptomics.Curr Issues Mol Biol. 2021;42:223-266. doi: 10.21775/cimb.042.223. Epub 2020 Dec 10. Curr Issues Mol Biol. 2021. PMID: 33300497 Free PMC article.
References
-
- Adams D. A., Gallagher K. M., Jajosky R. A., Kriseman J., Sharp P., Anderson W. J., et al. (2013). Summary of notifiable diseases—United States, 2011. MMWR Morb. Mortal. Wkly. Rep. 60, 1–117 - PubMed
-
- Adams D. A., Gallagher K. M., Jajosky R. A., Ward J., Sharp P., Anderson W. J., et al. (2012). Summary of notifiable diseases—United States, 2010. MMWR Morb. Mortal. Wkly. Rep. 59, 1–111 - PubMed
-
- Andrade M. O., Alegria M. C., Guzzo C. R., Docena C., Rosa M. C., Ramos C. H., et al. (2006). The HD-GYP domain of RpfG mediates a direct linkage between the Rpf quorum-sensing pathway and a subset of diguanylate cyclase proteins in the phytopathogen Xanthomonas axonopodis pv. citri. Mol. Microbiol. 62, 537–551 10.1111/j.1365-2958.2006.05386.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

