Cancer wars: natural products strike back
- PMID: 24822174
- PMCID: PMC4013484
- DOI: 10.3389/fchem.2014.00020
Cancer wars: natural products strike back
Abstract
Natural products have historically been a mainstay source of anticancer drugs, but in the 90's they fell out of favor in pharmaceutical companies with the emergence of targeted therapies, which rely on antibodies or small synthetic molecules identified by high throughput screening. Although targeted therapies greatly improved the treatment of a few cancers, the benefit has remained disappointing for many solid tumors, which revitalized the interest in natural products. With the approval of rapamycin in 2007, 12 novel natural product derivatives have been brought to market. The present review describes the discovery and development of these new anticancer drugs and highlights the peculiarities of natural product and new trends in this exciting field of drug discovery.
Keywords: cancer; drug discovery; molecular targets; natural products; pharmacognosy; privileged structures.
Figures
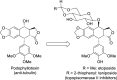
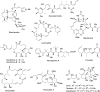
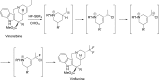

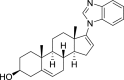

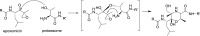
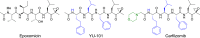
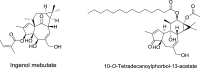
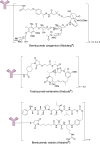

References
-
- Aicher T. D., Buszek K. R., Fang F. G., Forsyth C. J., Jung S. H., Kishi Y., et al. (1992). Total synthesis of halichondrin B and norhalichondrin B. J. Am. Chem. Soc. 114, 3162–3164 10.1021/ja00034a086 - DOI
-
- Ares J., Durán-Peña M. J., Hernández-Galán R., Collado I. (2013). Chemical genetics strategies for identification of molecular targets. Phytochem. Rev. 12, 895–914 10.1007/s11101-013-9312-6 - DOI
-
- Balog A., Meng D., Kamenecka T., Bertinato P., Su D.-S., Sorensen E. J., et al. (1996). Totalsynthese von (-)-Epothilon A. Angew. Chem. 108, 2976–2978 10.1002/ange.19961082318 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

