A two-step lyssavirus real-time polymerase chain reaction using degenerate primers with superior sensitivity to the fluorescent antigen test
- PMID: 24822188
- PMCID: PMC4009295
- DOI: 10.1155/2014/256175
A two-step lyssavirus real-time polymerase chain reaction using degenerate primers with superior sensitivity to the fluorescent antigen test
Abstract
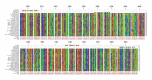
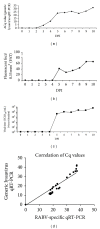
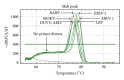
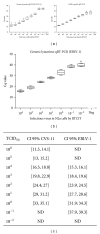
A generic two-step lyssavirus real-time reverse transcriptase polymerase chain reaction (qRT-PCR), based on a nested PCR strategy, was validated for the detection of different lyssavirus species. Primers with 17 to 30% of degenerate bases were used in both consecutive steps. The assay could accurately detect RABV, LBV, MOKV, DUVV, EBLV-1, EBLV-2, and ABLV. In silico sequence alignment showed a functional match with the remaining lyssavirus species. The diagnostic specificity was 100% and the sensitivity proved to be superior to that of the fluorescent antigen test. The limit of detection was ≤ 1 50% tissue culture infectious dose. The related vesicular stomatitis virus was not recognized, confirming the selectivity for lyssaviruses. The assay was applied to follow the evolution of rabies virus infection in the brain of mice from 0 to 10 days after intranasal inoculation. The obtained RNA curve corresponded well with the curves obtained by a one-step monospecific RABV-qRT-PCR, the fluorescent antigen test, and virus titration. Despite the presence of degenerate bases, the assay proved to be highly sensitive, specific, and reproducible.
Figures
References
-
- Kuzmin IV, Hughes GJ, Botvinkin AD, Orciari LA, Rupprecht CE. Phylogenetic relationships of Irkut and West Caucasian bat viruses within the Lyssavirus genus and suggested quantitative criteria based on the N gene sequence for Lyssavirus genotype definition. Virus Research. 2005;111(1):28–43. - PubMed
-
- Freuling CM, Abendroth B, Beer M, et al. Molecular diagnostics for the detection of Bokeloh bat Lyssavirus in a bat from Bavaria, Germany. Virus Research. 2013;177(2):201–204. - PubMed
-
- Johnson N, Vos A, Freuling C, Tordo N, Fooks AR, Müller T. Human rabies due to Lyssavirus infection of bat origin. Veterinary Microbiology. 2010;142(3-4):151–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

