Soybean seeds: a practical host for the production of functional subunit vaccines
- PMID: 24822195
- PMCID: PMC4005145
- DOI: 10.1155/2014/340804
Soybean seeds: a practical host for the production of functional subunit vaccines
Abstract
Soybean seeds possess several inherent qualities that make them an ideal host for the production of biopharmaceuticals when compared with other plant-based and non-plant-based recombinant expression systems (e.g., low cost of production, high protein to biomass ratio, long-term stability of seed proteins under ambient conditions, etc.). To demonstrate the practicality and feasibility of this platform for the production of subunit vaccines, we chose to express and characterize a nontoxic form of S. aureus enterotoxin B (mSEB) as a model vaccine candidate. We show that soy-mSEB was produced at a high vaccine to biomass ratio and represented ~76 theoretical doses of human vaccine per single soybean seed. We localized the model vaccine candidate both intracellularly and extracellularly and found no difference in mSEB protein stability or accumulation relative to subcellular environment. We also show that the model vaccine was biochemically and immunologically similar to native and recombinant forms of the protein produced in a bacterial expression system. Immunization of mice with seed extracts containing mSEB mounted a significant immune response within 14 days of the first injection. Taken together, our results highlight the practicality of soybean seeds as a potential platform for the production of functional subunit vaccines.
Figures

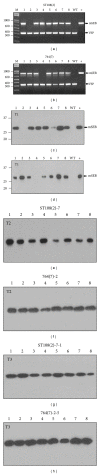

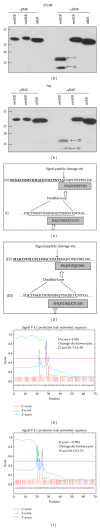


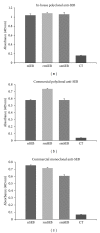
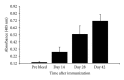
Similar articles
-
Accumulation of functional recombinant human coagulation factor IX in transgenic soybean seeds.Transgenic Res. 2011 Aug;20(4):841-55. doi: 10.1007/s11248-010-9461-y. Epub 2010 Nov 11. Transgenic Res. 2011. PMID: 21069460
-
Sublethal staphylococcal enterotoxin B challenge model in pigs to evaluate protection following immunization with a soybean-derived vaccine.Clin Vaccine Immunol. 2013 Jan;20(1):24-32. doi: 10.1128/CVI.00526-12. Epub 2012 Oct 31. Clin Vaccine Immunol. 2013. PMID: 23114702 Free PMC article.
-
Expression of functional recombinant human growth hormone in transgenic soybean seeds.Transgenic Res. 2011 Aug;20(4):811-26. doi: 10.1007/s11248-010-9460-z. Epub 2010 Nov 11. Transgenic Res. 2011. PMID: 21069461
-
Transgenic soybeans and soybean protein analysis: an overview.J Agric Food Chem. 2013 Dec 4;61(48):11736-43. doi: 10.1021/jf402148e. Epub 2013 Oct 24. J Agric Food Chem. 2013. PMID: 24099420 Review.
-
Expression and characterisation of recombinant molecules in transgenic soybean.Curr Pharm Des. 2013;19(31):5553-63. doi: 10.2174/1381612811319310010. Curr Pharm Des. 2013. PMID: 23394558 Review.
Cited by
-
A Comparison of transgenic and wild type soybean seeds: analysis of transcriptome profiles using RNA-Seq.BMC Biotechnol. 2015 Oct 1;15:89. doi: 10.1186/s12896-015-0207-z. BMC Biotechnol. 2015. PMID: 26427366 Free PMC article.
-
Systemic cytokine and chemokine responses in immunized mice challenged with staphylococcal enterotoxin B.Toxicon. 2017 Jul;133:82-90. doi: 10.1016/j.toxicon.2017.05.005. Epub 2017 May 3. Toxicon. 2017. PMID: 28478060 Free PMC article.
-
Seed-Based System for Cost-Effective Production of Vaccine Against Chronic Respiratory Disease in Chickens.Mol Biotechnol. 2023 Apr;65(4):570-580. doi: 10.1007/s12033-022-00554-5. Epub 2022 Sep 10. Mol Biotechnol. 2023. PMID: 36087216 Free PMC article.
-
Elastin-like polypeptide and γ-zein fusions significantly increase recombinant protein accumulation in soybean seeds.Transgenic Res. 2021 Oct;30(5):675-686. doi: 10.1007/s11248-021-00258-7. Epub 2021 May 8. Transgenic Res. 2021. PMID: 33963986
-
High accumulation in tobacco seeds of hemagglutinin antigen from avian (H5N1) influenza.Transgenic Res. 2017 Dec;26(6):775-789. doi: 10.1007/s11248-017-0047-9. Epub 2017 Oct 6. Transgenic Res. 2017. PMID: 28986672
References
-
- Boothe J, Nykiforuk C, Shen Y, et al. Seed-based expression systems for plant molecular farming. Plant Biotechnology Journal. 2010;8(5):588–606. - PubMed
-
- Rai M, Padh H. Expression systems for production of heterologous proteins. Current Science. 2001;80(9):1121–1128.
-
- Brondyk WH. Selecting an appropriate method for expressing a recombinant protein. Methods in Enzymology. 2009;463:131–147. - PubMed
-
- Hood EE, Witcher DR, Maddock S, et al. Commercial production of avidin from transgenic maizecharacterization of transformant, production, processing, extraction and purification. Molecular Breeding. 1997;3(4):291–306.
-
- Powell R, Hudson LC, Lambirth KC, et al. Recombinant expression of homodimeric 660 kDa human thyroglobulin in soybean seeds: an alternative source of human thyroglobulin. Plant Cell Reports. 2011;30(7):1327–1338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

