Topical nepafenac in treatment of acute central serous chorioretinopathy
- PMID: 24822228
- PMCID: PMC4017634
Topical nepafenac in treatment of acute central serous chorioretinopathy
Abstract
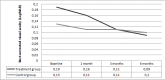
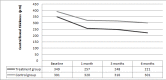
This study had been performed to investigate the anatomic and functional outcomes of nepafenac 0.1% therapy in acute central serous chorioretinopathy (CSC). The medical records of 30 patients with acute CSC were reviewed for a total of 31 eye charts. Seventeen eye records of 16 patients who were treated with topical nepafenac 0.1% three times daily for four weeks and continued until complete resolution of subretinal fluid were appraised. Fourteen patients with acute CSC (a total of 14 eye records) who did not receive treatment served as the control group also had been recorded. The proportion of eyes with complete resolution of subretinal fluid, serial changes in the mean best corrected visual acuity (BCVA), and the mean central foveal thickness (CFT) at 6 months of therapy were the outcomes measured. Mean age was 42.6±8.2 years in the treatment group and 41.1±7.1 years in the control group (p=0.85). At 6 months, 14 eyes (82.3%) in the treatment group and 6 eyes (42.8%) in the control group revealed a complete resolution in the subretinal fluid (p=0.02). In the treatment group, mean BCVA (LogMAR) significantly improved from 0.19±0.17 at baseline to 0.09±0.12 at 6 months (p=0.01). In the control group, mean BCVA (LogMAR) was 0.13±0.14 at baseline and decreased to 0.1±0.11 at 6 months (p=0.28). In the treatment group, mean CFT was 349±115 µm at baseline and significantly improved to 221±95 µm at 6 months (p<0.01). In the control group, mean CFT declined from 391±138 µm at baseline to 301±125 µm at 6 months (p=0.06). No treatment-related ocular or systemic side effects were observed. In conclusion, nepafenac 0.1% has the potential to treatment acute CSC. Further trials are warranted to study its safety and efficacy for this disease.
Keywords: Acute Central Serous Chorioretinopathy; Central Foveal Thickness; LogMAR; Subretinal Fluid; Topical Nepafenac; Visual Acuity.
Figures
References
-
- Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994;14(3):231–42. PMID: 7973118. - PubMed
-
- Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. ActaOphthalmol. 2008 Mar;86(2):126–45. PMID: 17662099. - PubMed
-
- Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989 Jun;96(6):854–9. PMID: 2740080. - PubMed
-
- Loo RH, Scott IU, Flynn HW Jr, Gass JD, Murray TG, Lewis ML, Rosenfeld PJ, Smiddy WE. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002 Feb;22(1):19–24. PMID: 11884873. - PubMed
LinkOut - more resources
Full Text Sources