Priming of protein expression in the defence response of Zantedeschia aethiopica to Pectobacterium carotovorum
- PMID: 24822269
- PMCID: PMC6638884
- DOI: 10.1111/mpp.12100
Priming of protein expression in the defence response of Zantedeschia aethiopica to Pectobacterium carotovorum
Abstract
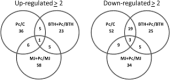
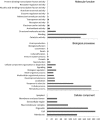
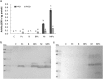
The defence response of Zantedeschia aethiopica, a natural rhizomatous host of the soft rot bacterium Pectobacterium carotovorum, was studied following the activation of common induced resistance pathways—systemic acquired resistance and induced systemic resistance. Proteomic tools were used, together with in vitro quantification and in situ localization of selected oxidizing enzymes. In total, 527 proteins were analysed by label-free mass spectrometry (MS) and annotated against the National Center for Biotechnology Information (NCBI) nonredundant (nr) protein database of rice (Oryza sativa). Of these, the fore most differentially expressed group comprised 215 proteins that were primed following application of methyl jasmonate (MJ) and subsequent infection with the pathogen. Sixty-five proteins were down-regulated following MJ treatments. The application of benzothiadiazole (BTH) increased the expression of 23 proteins; however, subsequent infection with the pathogen repressed their expression and did not induce priming. The sorting of primed proteins by Gene Ontology protein function category revealed that the primed proteins included nucleic acid-binding proteins, cofactor-binding proteins, ion-binding proteins, transferases, hydrolases and oxidoreductases. In line with the highlighted involvement of oxidoreductases in the defence response, we determined their activities, priming pattern and localization in planta. Increased activities were confined to the area surrounding the pathogen penetration site, associating these enzymes with the induced systemic resistance afforded by the jasmonic acid signalling pathway. The results presented here demonstrate the concerted priming of protein expression following MJ treatment, making it a prominent part of the defence response of Z. aethiopica to P. carotovorum.
Figures


Similar articles
-
Characterization of Pectobacterium carotovorum proteins differentially expressed during infection of Zantedeschia elliotiana in vivo and in vitro which are essential for virulence.Mol Plant Pathol. 2018 Jan;19(1):35-48. doi: 10.1111/mpp.12493. Epub 2016 Nov 23. Mol Plant Pathol. 2018. PMID: 27671364 Free PMC article.
-
A systemic response of geophytes is demonstrated by patterns of protein expression and the accumulation of signal molecules in Zantedeschia aethiopica.Plant Physiol Biochem. 2013 Oct;71:218-25. doi: 10.1016/j.plaphy.2013.07.014. Epub 2013 Aug 7. Plant Physiol Biochem. 2013. PMID: 23968930
-
Priming of antimicrobial phenolics during induced resistance response towards Pectobacterium carotovorum in the ornamental monocot calla lily.J Agric Food Chem. 2007 Dec 12;55(25):10315-22. doi: 10.1021/jf072037+. Epub 2007 Nov 10. J Agric Food Chem. 2007. PMID: 17994692
-
Priming of the Arabidopsis pattern-triggered immunity response upon infection by necrotrophic Pectobacterium carotovorum bacteria.Mol Plant Pathol. 2013 Jan;14(1):58-70. doi: 10.1111/j.1364-3703.2012.00827.x. Epub 2012 Sep 4. Mol Plant Pathol. 2013. PMID: 22947164 Free PMC article.
-
Rotting softly and stealthily.Curr Opin Plant Biol. 2005 Aug;8(4):424-9. doi: 10.1016/j.pbi.2005.04.001. Curr Opin Plant Biol. 2005. PMID: 15970273 Review.
Cited by
-
β-Aminobutyric Acid Priming Acquisition and Defense Response of Mango Fruit to Colletotrichum gloeosporioides Infection Based on Quantitative Proteomics.Cells. 2019 Sep 4;8(9):1029. doi: 10.3390/cells8091029. Cells. 2019. PMID: 31487826 Free PMC article.
-
A Switch from Latent to Typical Infection during Pectobacterium atrosepticum-Tobacco Interactions: Predicted and True Molecular Players.Int J Mol Sci. 2023 Aug 27;24(17):13283. doi: 10.3390/ijms241713283. Int J Mol Sci. 2023. PMID: 37686094 Free PMC article.
-
Quantitative Proteomics Reveals the Defense Response of Wheat against Puccinia striiformis f. sp. tritici.Sci Rep. 2016 Sep 28;6:34261. doi: 10.1038/srep34261. Sci Rep. 2016. PMID: 27678307 Free PMC article.
-
Characterization of Pectobacterium carotovorum proteins differentially expressed during infection of Zantedeschia elliotiana in vivo and in vitro which are essential for virulence.Mol Plant Pathol. 2018 Jan;19(1):35-48. doi: 10.1111/mpp.12493. Epub 2016 Nov 23. Mol Plant Pathol. 2018. PMID: 27671364 Free PMC article.
-
Transcriptome- Assisted Label-Free Quantitative Proteomics Analysis Reveals Novel Insights into Piper nigrum-Phytophthora capsici Phytopathosystem.Front Plant Sci. 2016 Jun 20;7:785. doi: 10.3389/fpls.2016.00785. eCollection 2016. Front Plant Sci. 2016. PMID: 27379110 Free PMC article.
References
-
- Afroz, A. , Khan, M.R. and Komatsu, S. (2010) Determination of proteins induced in response to jasmonic acid and salicylic acid in resistant and susceptible cultivars of tomato. Protein Pept. Lett. 17, 836–846. - PubMed
-
- Agrawal, G.K. and Rakwal, R. (2006) Rice proteomics: a cornerstone for cereal food crop proteomes. Mass Spectrom. Rev. 25, 1–53. - PubMed
-
- Baker, P.J. , Waugh, M.L. , Wang, X.G. , Stillman, T.J. , Turnbull, A.P. and Engel, P.C. (1997) Determinants of substrate specificity in the superfamily of amino acid dehydrogenases. Biochemistry, 36, 16 109–16 115. - PubMed
-
- Bolton, M.D. (2009) Primary metabolism and plant defense—fuel for the fire. Mol. Plant–Microbe Interact. 22, 487–497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

