Impairment of visual function and retinal ER stress activation in Wfs1-deficient mice
- PMID: 24823368
- PMCID: PMC4019519
- DOI: 10.1371/journal.pone.0097222
Impairment of visual function and retinal ER stress activation in Wfs1-deficient mice
Abstract
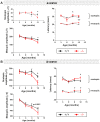
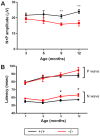
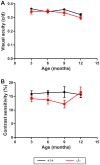
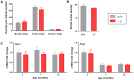
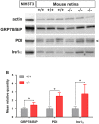
Wolfram syndrome is an early onset genetic disease (1/180,000) featuring diabetes mellitus and optic neuropathy, associated to mutations in the WFS1 gene. Wfs1-/- mouse model shows pancreatic beta cell atrophy, but its visual performance has not been investigated, prompting us to study its visual function and histopathology of the retina and optic nerve. Electroretinogram and visual evoked potentials (VEPs) were performed in Wfs1-/- and Wfs1+/+ mice at 3, 6, 9 and 12 months of age. Fundi were pictured with Micron III apparatus. Retinal ganglion cell (RGC) abundance was determined from Brn3a immunolabeling of retinal sections. RGC axonal loss was quantified by electron microscopy in transversal optic nerve sections. Endoplasmic reticulum stress was assessed using immunoglobulin binding protein (BiP), protein disulfide isomerase (PDI) and inositol-requiring enzyme 1 alpha (Ire1α) markers. Electroretinograms amplitudes were slightly reduced and latencies increased with time in Wfs1-/- mice. Similarly, VEPs showed decreased N+P amplitudes and increased N-wave latency. Analysis of unfolded protein response signaling revealed an activation of endoplasmic reticulum stress in Wfs1-/- mutant mouse retinas. Altogether, progressive VEPs alterations with minimal neuronal cell loss suggest functional alteration of the action potential in the Wfs1-/- optic pathways.
Conflict of interest statement
Figures


References
-
- Barrett TG, Bundey SE, Macleod AF (1995) Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet 346: 1458–1463. - PubMed
-
- Barrett TG, Bundey SE, Fielder AR, Good PA (1997) Optic atrophy in Wolfram (DIDMOAD) syndrome. Eye (Lond) 11 (Pt 6): 882–888. - PubMed
-
- Rando RA, Horton JC, Layzer RB (1992) Wolfram syndrome: evidence of a diffuse neurodegenerative disease by magnetic resonance imaging. Neurology 42: 1220–1224. - PubMed
-
- Wolfram DJ, Wagener HP (1938) Diabetes mellitus and simple optic atrophy among siblings: report of four cases. Mayo Clin Proc 9: 715–718.
-
- Swift RG, Polymeropoulos MH, Torres R, Swift M (1998) Predisposition of Wolfram syndrome heterozygotes to psychiatric illness. Mol Psychiatry 3: 86–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

