The chemokine receptor CXCR6 is required for the maintenance of liver memory CD8⁺ T cells specific for infectious pathogens
- PMID: 24823625
- PMCID: PMC4207865
- DOI: 10.1093/infdis/jiu281
The chemokine receptor CXCR6 is required for the maintenance of liver memory CD8⁺ T cells specific for infectious pathogens
Abstract
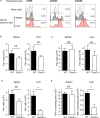
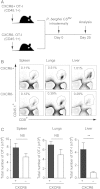
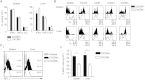
It is well established that immunization with attenuated malaria sporozoites induces CD8(+) T cells that eliminate parasite-infected hepatocytes. Liver memory CD8(+) T cells induced by immunization with parasites undergo a unique differentiation program and have enhanced expression of CXCR6. Following immunization with malaria parasites, CXCR6-deficient memory CD8(+) T cells recovered from the liver display altered cell-surface expression markers as compared to their wild-type counterparts, but they exhibit normal cytokine secretion and expression of cytotoxic mediators on a per-cell basis. Most importantly, CXCR6-deficient CD8(+) T cells migrate to the liver normally after immunization with Plasmodium sporozoites or vaccinia virus, but a few weeks later their numbers severely decrease in this organ, losing their capacity to inhibit malaria parasite development in the liver. These studies are the first to show that CXCR6 is critical for the development and maintenance of protective memory CD8(+) T cells in the liver.
Keywords: Plasmodium; chemokine receptor; liver; malaria; memory CD8+ T cells.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
From the draining lymph node to the liver: the induction and effector mechanisms of malaria-specific CD8+ T cells.Semin Immunopathol. 2015 May;37(3):211-20. doi: 10.1007/s00281-015-0479-3. Epub 2015 Apr 28. Semin Immunopathol. 2015. PMID: 25917387 Free PMC article. Review.
-
Long term protection after immunization with P. berghei sporozoites correlates with sustained IFNγ responses of hepatic CD8+ memory T cells.PLoS One. 2012;7(5):e36508. doi: 10.1371/journal.pone.0036508. Epub 2012 May 1. PLoS One. 2012. PMID: 22563506 Free PMC article.
-
Memory phenotype CD8(+) T cells persist in livers of mice protected against malaria by immunization with attenuated Plasmodium berghei sporozoites.Eur J Immunol. 1999 Dec;29(12):3978-86. doi: 10.1002/(SICI)1521-4141(199912)29:12<3978::AID-IMMU3978>3.0.CO;2-0. Eur J Immunol. 1999. PMID: 10602007
-
Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes.J Immunol. 2009 Nov 1;183(9):5870-8. doi: 10.4049/jimmunol.0900302. Epub 2009 Oct 7. J Immunol. 2009. PMID: 19812194
-
The role of intrahepatic lymphocytes in mediating protective immunity induced by attenuated Plasmodium berghei sporozoites.Immunol Rev. 2000 Apr;174:123-34. doi: 10.1034/j.1600-0528.2002.00013h.x. Immunol Rev. 2000. PMID: 10807512 Review.
Cited by
-
From the draining lymph node to the liver: the induction and effector mechanisms of malaria-specific CD8+ T cells.Semin Immunopathol. 2015 May;37(3):211-20. doi: 10.1007/s00281-015-0479-3. Epub 2015 Apr 28. Semin Immunopathol. 2015. PMID: 25917387 Free PMC article. Review.
-
Functional Heterogeneity of CD4+ Tumor-Infiltrating Lymphocytes With a Resident Memory Phenotype in NSCLC.Front Immunol. 2018 Nov 16;9:2654. doi: 10.3389/fimmu.2018.02654. eCollection 2018. Front Immunol. 2018. PMID: 30505306 Free PMC article.
-
mRNA vaccine against malaria tailored for liver-resident memory T cells.Nat Immunol. 2023 Sep;24(9):1487-1498. doi: 10.1038/s41590-023-01562-6. Epub 2023 Jul 20. Nat Immunol. 2023. PMID: 37474653
-
Systemic virus infection results in CD8 T cell recruitment to the retina in the absence of local virus infection.Front Immunol. 2023 Aug 18;14:1221511. doi: 10.3389/fimmu.2023.1221511. eCollection 2023. Front Immunol. 2023. PMID: 37662932 Free PMC article.
-
Evolutionary diversity of CXCL16-CXCR6: Convergent substitutions and recurrent gene loss in sauropsids.Immunogenetics. 2024 Dec;76(5-6):397-415. doi: 10.1007/s00251-024-01357-5. Epub 2024 Oct 14. Immunogenetics. 2024. PMID: 39400711
References
-
- Seder RA, Chang L-J, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–65. - PubMed
-
- Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13:1035–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

