Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia
- PMID: 24823639
- PMCID: PMC4072043
- DOI: 10.1016/j.ccr.2014.03.014
Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia
Abstract
Many human cancers are dramatically accelerated by chronic inflammation. However, the specific cellular and molecular elements mediating this effect remain largely unknown. Using a murine model of pancreatic intraepithelial neoplasia (PanIN), we found that Kras(G12D) induces expression of functional IL-17 receptors on PanIN epithelial cells and also stimulates infiltration of the pancreatic stroma by IL-17-producing immune cells. Both effects are augmented by associated chronic pancreatitis, resulting in functional in vivo changes in PanIN epithelial gene expression. Forced IL-17 overexpression dramatically accelerates PanIN initiation and progression, while inhibition of IL-17 signaling using genetic or pharmacologic techniques effectively prevents PanIN formation. Together, these studies suggest that a hematopoietic-to-epithelial IL-17 signaling axis is a potent and requisite driver of PanIN formation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
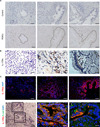
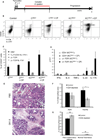
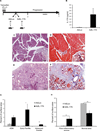
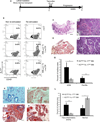

Comment in
-
Tumor-promoting inflammatory networks in pancreatic neoplasia: another reason to loathe Kras.Cancer Cell. 2014 May 12;25(5):553-4. doi: 10.1016/j.ccr.2014.04.020. Cancer Cell. 2014. PMID: 24823632 Free PMC article.
-
Cancer: Targeting IL-17 in pancreatic cancer.Nat Rev Drug Discov. 2014 Jul;13(7):493. doi: 10.1038/nrd4372. Nat Rev Drug Discov. 2014. PMID: 24981357 No abstract available.
Similar articles
-
Tumor-promoting inflammatory networks in pancreatic neoplasia: another reason to loathe Kras.Cancer Cell. 2014 May 12;25(5):553-4. doi: 10.1016/j.ccr.2014.04.020. Cancer Cell. 2014. PMID: 24823632 Free PMC article.
-
Inhibition of chronic pancreatitis and pancreatic intraepithelial neoplasia (PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice.Carcinogenesis. 2011 Nov;32(11):1689-96. doi: 10.1093/carcin/bgr191. Epub 2011 Aug 22. Carcinogenesis. 2011. PMID: 21859833 Free PMC article.
-
Trisomy of the Dscr1 gene suppresses early progression of pancreatic intraepithelial neoplasia driven by oncogenic Kras.Biochem Biophys Res Commun. 2013 Oct 11;440(1):50-5. doi: 10.1016/j.bbrc.2013.09.033. Epub 2013 Sep 13. Biochem Biophys Res Commun. 2013. PMID: 24041692
-
Critical role of oncogenic KRAS in pancreatic cancer (Review).Mol Med Rep. 2016 Jun;13(6):4943-9. doi: 10.3892/mmr.2016.5196. Epub 2016 Apr 27. Mol Med Rep. 2016. PMID: 27121414 Review.
-
Unveiling the role of interleukin-6 in pancreatic cancer occurrence and progression.Front Endocrinol (Lausanne). 2024 May 17;15:1408312. doi: 10.3389/fendo.2024.1408312. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38828409 Free PMC article. Review.
Cited by
-
Immune checkpoint blockade in pancreatic cancer: Trudging through the immune desert.Semin Cancer Biol. 2022 Nov;86(Pt 2):14-27. doi: 10.1016/j.semcancer.2022.08.009. Epub 2022 Aug 27. Semin Cancer Biol. 2022. PMID: 36041672 Free PMC article. Review.
-
CRISPR/Cas9 therapeutics: a cure for cancer and other genetic diseases.Oncotarget. 2016 Aug 9;7(32):52541-52552. doi: 10.18632/oncotarget.9646. Oncotarget. 2016. PMID: 27250031 Free PMC article. Review.
-
Daxx maintains endogenous retroviral silencing and restricts cellular plasticity in vivo.Sci Adv. 2020 Aug 5;6(32):eaba8415. doi: 10.1126/sciadv.aba8415. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32821827 Free PMC article.
-
RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer.Cancer Cell. 2018 Nov 12;34(5):757-774.e7. doi: 10.1016/j.ccell.2018.10.006. Cancer Cell. 2018. PMID: 30423296 Free PMC article.
-
The role of interleukin-17 in tumor development and progression.J Exp Med. 2020 Jan 6;217(1):e20190297. doi: 10.1084/jem.20190297. J Exp Med. 2020. PMID: 31727782 Free PMC article. Review.
References
-
- Bailey JM, Leach SD. Signaling pathways mediating epithelial- mesenchymal crosstalk in pancreatic cancer: Hedgehog, Notch and TGFbeta. In: Grippo PJ, Munshi HG, editors. Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India): 2012. - PubMed
-
- Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, Putney L, Ferrick DA, Hyde DM, Love RB. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 2008;31:167–179. - PubMed
-
- Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, Batra SK. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Molecular cancer research : MCR. 2007;5:309–320. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

