RNA mimicry by the fap7 adenylate kinase in ribosome biogenesis
- PMID: 24823650
- PMCID: PMC4019466
- DOI: 10.1371/journal.pbio.1001860
RNA mimicry by the fap7 adenylate kinase in ribosome biogenesis
Abstract
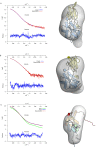
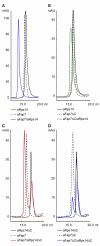
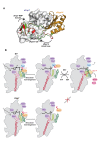
During biogenesis of the 40S and 60S ribosomal subunits, the pre-40S particles are exported to the cytoplasm prior to final cleavage of the 20S pre-rRNA to mature 18S rRNA. Amongst the factors involved in this maturation step, Fap7 is unusual, as it both interacts with ribosomal protein Rps14 and harbors adenylate kinase activity, a function not usually associated with ribonucleoprotein assembly. Human hFap7 also regulates Cajal body assembly and cell cycle progression via the p53-MDM2 pathway. This work presents the functional and structural characterization of the Fap7-Rps14 complex. We report that Fap7 association blocks the RNA binding surface of Rps14 and, conversely, Rps14 binding inhibits adenylate kinase activity of Fap7. In addition, the affinity of Fap7 for Rps14 is higher with bound ADP, whereas ATP hydrolysis dissociates the complex. These results suggest that Fap7 chaperones Rps14 assembly into pre-40S particles via RNA mimicry in an ATP-dependent manner. Incorporation of Rps14 by Fap7 leads to a structural rearrangement of the platform domain necessary for the pre-rRNA to acquire a cleavage competent conformation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
An open interface in the pre-80S ribosome coordinated by ribosome assembly factors Tsr1 and Dim1 enables temporal regulation of Fap7.RNA. 2021 Feb;27(2):221-233. doi: 10.1261/rna.077610.120. Epub 2020 Nov 20. RNA. 2021. PMID: 33219089 Free PMC article.
-
The putative NTPase Fap7 mediates cytoplasmic 20S pre-rRNA processing through a direct interaction with Rps14.Mol Cell Biol. 2005 Dec;25(23):10352-64. doi: 10.1128/MCB.25.23.10352-10364.2005. Mol Cell Biol. 2005. PMID: 16287850 Free PMC article.
-
Essential ribosome assembly factor Fap7 regulates a hierarchy of RNA-protein interactions during small ribosomal subunit biogenesis.Proc Natl Acad Sci U S A. 2013 Sep 17;110(38):15253-8. doi: 10.1073/pnas.1306389110. Epub 2013 Sep 3. Proc Natl Acad Sci U S A. 2013. PMID: 24003121 Free PMC article.
-
Inside the 40S ribosome assembly machinery.Curr Opin Chem Biol. 2011 Oct;15(5):657-63. doi: 10.1016/j.cbpa.2011.07.023. Epub 2011 Aug 20. Curr Opin Chem Biol. 2011. PMID: 21862385 Free PMC article. Review.
-
Nuclear export and cytoplasmic maturation of ribosomal subunits.FEBS Lett. 2007 Jun 19;581(15):2783-93. doi: 10.1016/j.febslet.2007.05.013. Epub 2007 May 11. FEBS Lett. 2007. PMID: 17509569 Review.
Cited by
-
MoFap7, a ribosome assembly factor, is required for fungal development and plant colonization of Magnaporthe oryzae.Virulence. 2019 Dec;10(1):1047-1063. doi: 10.1080/21505594.2019.1697123. Virulence. 2019. PMID: 31814506 Free PMC article.
-
Ribosome assembly factor Adenylate Kinase 6 maintains cell proliferation and cell size homeostasis during root growth.New Phytol. 2020 Mar;225(5):2064-2076. doi: 10.1111/nph.16291. Epub 2019 Dec 2. New Phytol. 2020. PMID: 31665812 Free PMC article.
-
Rio1 mediates ATP-dependent final maturation of 40S ribosomal subunits.Nucleic Acids Res. 2014 Oct 29;42(19):12189-99. doi: 10.1093/nar/gku878. Epub 2014 Oct 7. Nucleic Acids Res. 2014. PMID: 25294836 Free PMC article.
-
Structure of a human pre-40S particle points to a role for RACK1 in the final steps of 18S rRNA processing.Nucleic Acids Res. 2016 Sep 30;44(17):8465-78. doi: 10.1093/nar/gkw714. Epub 2016 Aug 16. Nucleic Acids Res. 2016. PMID: 27530427 Free PMC article.
-
Co-translational capturing of nascent ribosomal proteins by their dedicated chaperones.Nat Commun. 2015 Jun 26;6:7494. doi: 10.1038/ncomms8494. Nat Commun. 2015. PMID: 26112308 Free PMC article.
References
-
- Kressler D, Hurt E, Bassler J (2010) Driving ribosome assembly. Biochim Biophys Acta BBA - Mol Cell Res 1803: 673–683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

