Improving protocol design feasibility to drive drug development economics and performance
- PMID: 24823665
- PMCID: PMC4053871
- DOI: 10.3390/ijerph110505069
Improving protocol design feasibility to drive drug development economics and performance
Abstract
Protocol design complexity has increased substantially during the past decade and this in turn has adversely impacted drug development economics and performance. This article reviews the results of two major Tufts Center for the Study of Drug Development studies quantifying the direct cost of conducting less essential and unnecessary protocol procedures and of implementing amendments to protocol designs. Indirect costs including personnel time, work load and cycle time delays associated with complex protocol designs are also discussed. The author concludes with an overview of steps that research sponsors are taking to improve protocol design feasibility.
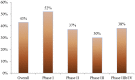
Figures
References
-
- Getz K., Zuckerman R., Cropp A., Hindle A., Krauss R., Kaitin K. Measuring the incidence, causes, and repercussions of protocol amendments. Drug Inf. J. 2011;45:265–275.
-
- Getz K., Wenger J., Campo R., Seguine E., Kaitin K. Assessing the impact of protocol design change on clinical trial performance. Amer. J. Ther. 2008;15:449–456. - PubMed
-
- Clark T. Data is King. [(accessed on 4 May 2014)]. Available online: http://www.samedanltd.com/magazine/13/issue/173/article/3242.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous