Inflammation programs self-reactive CD8+ T cells to acquire T-box-mediated effector function but does not prevent deletional tolerance
- PMID: 24823810
- PMCID: PMC4138202
- DOI: 10.1189/jlb.1A0913-500RR
Inflammation programs self-reactive CD8+ T cells to acquire T-box-mediated effector function but does not prevent deletional tolerance
Abstract
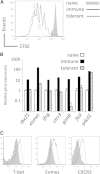
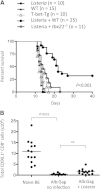
CD8(+) T cells must detect foreign antigens and differentiate into effector cells to eliminate infections. But, when self-antigen is recognized instead, mechanisms of peripheral tolerance prevent acquisition of effector function to avoid autoimmunity. These distinct responses are influenced by inflammatory and regulatory clues from the tissue environment, but the mechanism(s) by which naive T cells interpret these signals to generate the appropriate immune response are unclear. The identification of the molecules operative in these cell-fate decisions is crucial for developing new treatment options for patients with cancer or autoimmunity, where manipulation of T cell activity is desired to alter the course of disease. With the use of an in vivo murine model to examine CD8(+) T cell responses to healthy self-tissue, we correlated self-tolerance with a failure to induce the T-box transcription factors T-bet and Eomes. However, inflammation associated with acute microbial infection induced T-bet and Eomes expression and promoted effector differentiation of self-reactive T cells under conditions that normally favor tolerance. In the context of a Listeria infection, these functional responses relied on elevated T-bet expression, independent of Eomes. Alternatively, infection with LCMV induced higher Eomes expression, which was sufficient in the absence of T-bet to promote effector cytokine production. Our results place T-box transcription factors at a molecular crossroads between CD8(+) T cell anergy and effector function upon recognition of peripheral self-antigen, and suggest that inflammation during T cell priming directs these distinct cellular responses.
Keywords: effector differentiation/immunity; immunotherapy; transcription factors.
© 2014 Society for Leukocyte Biology.
Figures






Similar articles
-
Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation.J Immunol. 2006 Dec 1;177(11):7515-9. doi: 10.4049/jimmunol.177.11.7515. J Immunol. 2006. PMID: 17114419
-
[Clonal deletion and clonal anergy as the mechanism of self-tolerance induction].Nihon Rinsho. 1997 Jun;55(6):1331-6. Nihon Rinsho. 1997. PMID: 9200914 Review. Japanese.
-
Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection.Curr Top Microbiol Immunol. 2005;293:43-71. doi: 10.1007/3-540-27702-1_3. Curr Top Microbiol Immunol. 2005. PMID: 15981475 Review.
-
IRF4 Regulates the Ratio of T-Bet to Eomesodermin in CD8+ T Cells Responding to Persistent LCMV Infection.PLoS One. 2015 Dec 29;10(12):e0144826. doi: 10.1371/journal.pone.0144826. eCollection 2015. PLoS One. 2015. PMID: 26714260 Free PMC article.
-
Signal 3 cytokines as modulators of primary immune responses during infections: the interplay of type I IFN and IL-12 in CD8 T cell responses.PLoS One. 2012;7(7):e40865. doi: 10.1371/journal.pone.0040865. Epub 2012 Jul 17. PLoS One. 2012. PMID: 22815848 Free PMC article.
Cited by
-
Natural Compounds with Potential to Modulate Cancer Therapies and Self-Reactive Immune Cells.Cancers (Basel). 2020 Mar 13;12(3):673. doi: 10.3390/cancers12030673. Cancers (Basel). 2020. PMID: 32183059 Free PMC article. Review.
-
Neuropilin-1 expression is induced on tolerant self-reactive CD8+ T cells but is dispensable for the tolerant phenotype.PLoS One. 2014 Oct 24;9(10):e110707. doi: 10.1371/journal.pone.0110707. eCollection 2014. PLoS One. 2014. PMID: 25343644 Free PMC article.
-
Checkpoint blockade immunotherapy relies on T-bet but not Eomes to induce effector function in tumor-infiltrating CD8+ T cells.Cancer Immunol Res. 2015 Feb;3(2):116-24. doi: 10.1158/2326-6066.CIR-14-0159. Epub 2014 Dec 16. Cancer Immunol Res. 2015. PMID: 25516478 Free PMC article.
-
Genome-Wide DNA Methylation Profiling in CD8 T-Cells and Gamma Delta T-Cells of Asian Indian Patients With Takayasu Arteritis.Front Cell Dev Biol. 2022 Jun 23;10:843413. doi: 10.3389/fcell.2022.843413. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35813204 Free PMC article.
-
Autoimmune Encephalitis in COVID-19 Infection: Our Experience and Systematic Review of the Literature.Biomedicines. 2022 Mar 25;10(4):774. doi: 10.3390/biomedicines10040774. Biomedicines. 2022. PMID: 35453524 Free PMC article. Review.
References
-
- Redmond W. L., Sherman L. A. (2005) Peripheral tolerance of CD8 T lymphocytes. Immunity 22, 275–284. - PubMed
-
- Ohlen C., Kalos M., Cheng L. E., Shur A. C., Hong D. J., Carson B. D., Kokot N. C., Lerner C. G., Sather B. D., Huseby E. S., Greenberg P. D. (2002) CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J. Exp. Med. 195, 1407–1418. - PMC - PubMed
-
- Berrien-Elliott M. M., Jackson S. R., Meyer J. M., Rouskey C. J., Nguyen T. L., Yagita H., Greenberg P., Dipaolo R. J., Teague R. M. (2013) Durable adoptive immunotherapy for leukemia produced by manipulation of multiple regulatory pathways of CD8+ T cell tolerance. Cancer Res. 73, 605–616. - PMC - PubMed
-
- Kaech S. M., Wherry E. J., Ahmed R. (2002) Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2, 251–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

