Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: perspective of anti-pigmenting agents
- PMID: 24823877
- PMCID: PMC4057732
- DOI: 10.3390/ijms15058293
Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: perspective of anti-pigmenting agents
Abstract
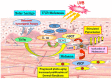
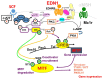
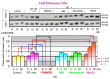
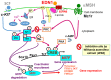
Few anti-pigmenting agents have been designed and developed according to their known hyperpigmentation mechanisms and corresponding intracellular signaling cascades. Most anti-pigmenting agents developed so far are mechanistically involved in the interruption of constitutional melanogenic mechanisms by which skin color is maintained at a normal and unstimulated level. Thus, owing to the difficulty of confining topical application to a specific hyperpigmented skin area, potent anti-pigmenting agents capable of attenuating the natural unstimulated pigmentation process have the risk of leading to hypopigmentation. Since intracellular signaling pathways within melanocytes do not function substantially in maintaining normal skin color and are activated only by environmental stimuli such as UV radiation, specifically down-regulating the activation of melanogenesis to the constitutive level would be an appropriate strategy to develop new potent anti-pigmenting agents with a low risk of hypopigmentation. In this article, we review the hyperpigmentation mechanisms and intracellular signaling pathways that lead to the stimulation of melanogenesis. We also discuss a screening and evaluation system to select candidates for new anti-melanogenic substances by focusing on inhibitors of endothelin-1 or stem cell factor-triggered intracellular signaling cascades. From this viewpoint, we show that extracts of the herbs Withania somnifera and Melia toosendan and the natural chemicals Withaferin A and Astaxanthin are new candidates for potent anti-pigmenting substances that avoid the risk of hypopigmentation.
Figures











Similar articles
-
An extract of Withania somnifera attenuates endothelin-1-stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes.Phytother Res. 2011 Sep;25(9):1398-411. doi: 10.1002/ptr.3552. Epub 2011 Jun 16. Phytother Res. 2011. PMID: 21678520
-
Anti-Pigmentation Effects of Eight Phellinus linteus-Fermented Traditional Crude Herbal Extracts on Brown Guinea Pigs of Ultraviolet B-Induced Hyperpigmentation.J Microbiol Biotechnol. 2018 Mar 28;28(3):375-380. doi: 10.4014/jmb.1711.11043. J Microbiol Biotechnol. 2018. PMID: 29316744
-
Plants as Modulators of Melanogenesis: Role of Extracts, Pure Compounds and Patented Compositions in Therapy of Pigmentation Disorders.Int J Mol Sci. 2022 Nov 26;23(23):14787. doi: 10.3390/ijms232314787. Int J Mol Sci. 2022. PMID: 36499134 Free PMC article. Review.
-
Astaxanthin and withaferin A block paracrine cytokine interactions between UVB-exposed human keratinocytes and human melanocytes via the attenuation of endothelin-1 secretion and its downstream intracellular signaling.Cytokine. 2015 Jun;73(2):184-97. doi: 10.1016/j.cyto.2015.02.006. Epub 2015 Mar 13. Cytokine. 2015. PMID: 25777483
-
Emerging Roles of Dermal Fibroblasts in Hyperpigmentation and Hypopigmentation: A Review.J Cosmet Dermatol. 2025 Jan;24(1):e16790. doi: 10.1111/jocd.16790. J Cosmet Dermatol. 2025. PMID: 39780507 Free PMC article. Review.
Cited by
-
Lactobacillus helveticus-Fermented Milk Whey Suppresses Melanin Production by Inhibiting Tyrosinase through Decreasing MITF Expression.Nutrients. 2020 Jul 14;12(7):2082. doi: 10.3390/nu12072082. Nutrients. 2020. PMID: 32674403 Free PMC article.
-
Therapeutic Potential of Fungal Terpenes and Terpenoids: Application in Skin Diseases.Molecules. 2024 Mar 6;29(5):1183. doi: 10.3390/molecules29051183. Molecules. 2024. PMID: 38474692 Free PMC article. Review.
-
Withaferin A-A Promising Phytochemical Compound with Multiple Results in Dermatological Diseases.Molecules. 2021 Apr 21;26(9):2407. doi: 10.3390/molecules26092407. Molecules. 2021. PMID: 33919088 Free PMC article. Review.
-
A Study of Efficacy and Safety of Ashwagandha (Withania somnifera) Lotion on Facial Skin in Photoaged Healthy Adults.Cureus. 2023 Mar 15;15(3):e36168. doi: 10.7759/cureus.36168. eCollection 2023 Mar. Cureus. 2023. PMID: 36937128 Free PMC article.
-
The Development of Sugar-Based Anti-Melanogenic Agents.Int J Mol Sci. 2016 Apr 16;17(4):583. doi: 10.3390/ijms17040583. Int J Mol Sci. 2016. PMID: 27092497 Free PMC article. Review.
References
-
- Chung K.W., Park Y.J., Choi Y.J., Park M.H., Ha Y.M., Uehara Y., Yoon J.H., Chun P., Moon H.R., Chung H.Y. Evaluation of in vitro and in vivo anti-melanogenic activity of a newly synthesized strong tyrosinase inhibitor (E)-3-(2,4-dihydroxybenzylidene) pyrrolidine-2,5-dione (3-DBP) Biochim. Biophys. Acta. 2012;1820:962–969. - PubMed
-
- Ando H., Funasaka Y., Oka M., Ohashi A., Furumura M., Matsunaga J., Matsunaga N., Hearing V.J., Ichihashi M. Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids on melanogenesis. J. Lipid Res. 1999;40:1312–1316. - PubMed
-
- Paine C., Sharlow E., Liebel F., Eisinger M., Shapiro S., Seiberg M. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J. Investig. Dermatol. 2001;116:587–595. - PubMed
-
- Yada Y., Higuchi K., Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J. Biol. Chem. 1991;266:18352–18357. - PubMed
-
- Imokawa G., Yada Y., Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J. Biol. Chem. 1992;267:24675–24680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

