Quantification of blood flow and topology in developing vascular networks
- PMID: 24823933
- PMCID: PMC4019654
- DOI: 10.1371/journal.pone.0096856
Quantification of blood flow and topology in developing vascular networks
Abstract
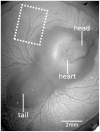
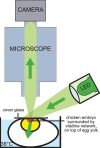
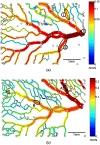
Since fluid dynamics plays a critical role in vascular remodeling, quantification of the hemodynamics is crucial to gain more insight into this complex process. Better understanding of vascular development can improve prediction of the process, and may eventually even be used to influence the vascular structure. In this study, a methodology to quantify hemodynamics and network structure of developing vascular networks is described. The hemodynamic parameters and topology are derived from detailed local blood flow velocities, obtained by in vivo micro-PIV measurements. The use of such detailed flow measurements is shown to be essential, as blood vessels with a similar diameter can have a large variation in flow rate. Measurements are performed in the yolk sacs of seven chicken embryos at two developmental stages between HH 13+ and 17+. A large range of flow velocities (1 µm/s to 1 mm/s) is measured in blood vessels with diameters in the range of 25-500 µm. The quality of the data sets is investigated by verifying the flow balances in the branching points. This shows that the quality of the data sets of the seven embryos is comparable for all stages observed, and the data is suitable for further analysis with known accuracy. When comparing two subsequently characterized networks of the same embryo, vascular remodeling is observed in all seven networks. However, the character of remodeling in the seven embryos differs and can be non-intuitive, which confirms the necessity of quantification. To illustrate the potential of the data, we present a preliminary quantitative study of key network topology parameters and we compare these with theoretical design rules.
Conflict of interest statement
Figures
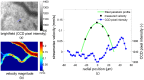
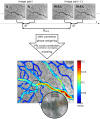
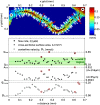
 . A selection is enlarged to show the corresponding time-averaged velocity vector field, illustrating the spatial resolution of the measurement results.
. A selection is enlarged to show the corresponding time-averaged velocity vector field, illustrating the spatial resolution of the measurement results.
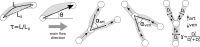
 , the angle between the vessel segment and the main flow direction
, the angle between the vessel segment and the main flow direction  , the arterial branching angle
, the arterial branching angle  , the venous branching angle
, the venous branching angle  , and Murray’s law ratio for both arterial and venous branches
, and Murray’s law ratio for both arterial and venous branches  and
and  .
.
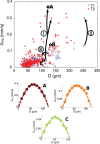
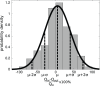
 close to zero and a small standard deviation
close to zero and a small standard deviation  .
.References
-
- Chapman W (1918) The effect of the heart-beat upon the development of the vascular system in the chick. Am J Anat 23: 175–203.
-
- Le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, et al. (2004) Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Dev 131: 361–375. - PubMed
-
- Nguyen T, Eichmann A, Le Noble F, Fleury V (2006) Dynamics of vascular branching morphogenesis: the effect of blood and tissue flow. Phys Rev E 73: 061907. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

