Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts
- PMID: 24823941
- PMCID: PMC4076082
- DOI: 10.1194/jlr.M048207
Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts
Abstract
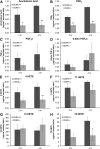
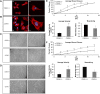
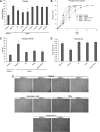
In these studies, the role of ceramide-1-phosphate (C1P) in the wound-healing process was investigated. Specifically, fibroblasts isolated from mice with the known anabolic enzyme for C1P, ceramide kinase (CERK), ablated (CERK(-/-) mice) and their wild-type littermates (CERK(+/+)) were subjected to in vitro wound-healing assays. Simulation of mechanical trauma of a wound by scratching a monolayer of fibroblasts from CERK(+/+) mice demonstrated steadily increasing levels of arachidonic acid in a time-dependent manner in stark contrast to CERK(-/-) fibroblasts. This observed difference was reflected in scratch-induced eicosanoid levels. Similar, but somewhat less intense, changes were observed in a more complex system utilizing skin biopsies obtained from CERK-null mice. Importantly, C1P levels increased during the early stages of human wound healing correlating with the transition from the inflammatory stage to the peak of the fibroplasia stage (e.g., proliferation and migration of fibroblasts). Finally, the loss of proper eicosanoid response translated into an abnormal migration pattern for the fibroblasts isolated from CERK(-/-) As the proper migration of fibroblasts is one of the necessary steps of wound healing, these studies demonstrate a novel requirement for the CERK-derived C1P in the proper healing response of wounds.
Keywords: ceramide-1-phosphate; lipidomics; wound healing.
Figures




Similar articles
-
Ceramide kinase regulates acute wound healing by suppressing 5-oxo-ETE biosynthesis and signaling via its receptor OXER1.J Lipid Res. 2022 Apr;63(4):100187. doi: 10.1016/j.jlr.2022.100187. Epub 2022 Feb 24. J Lipid Res. 2022. PMID: 35219746 Free PMC article.
-
Characterization of eicosanoid synthesis in a genetic ablation model of ceramide kinase.J Lipid Res. 2013 Jul;54(7):1834-47. doi: 10.1194/jlr.M035683. Epub 2013 Apr 10. J Lipid Res. 2013. PMID: 23576683 Free PMC article.
-
Regulation and traffic of ceramide 1-phosphate produced by ceramide kinase: comparative analysis to glucosylceramide and sphingomyelin.J Biol Chem. 2008 Mar 28;283(13):8517-26. doi: 10.1074/jbc.M707107200. Epub 2007 Dec 16. J Biol Chem. 2008. PMID: 18086664
-
Novel signaling aspects of ceramide 1-phosphate.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Apr;1865(4):158630. doi: 10.1016/j.bbalip.2020.158630. Epub 2020 Jan 17. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31958571 Review.
-
Ceramide kinase: the first decade.Cell Signal. 2011 Jun;23(6):999-1008. doi: 10.1016/j.cellsig.2010.11.012. Epub 2010 Nov 25. Cell Signal. 2011. PMID: 21111813 Review.
Cited by
-
Ceramide Kinase Inhibition Drives Ferroptosis and Sensitivity to Cisplatin in Mutant KRAS Lung Cancer by Dysregulating VDAC-Mediated Mitochondria Function.Mol Cancer Res. 2022 Sep 2;20(9):1429-1442. doi: 10.1158/1541-7786.MCR-22-0085. Mol Cancer Res. 2022. PMID: 35560154 Free PMC article.
-
Overexpression of acid ceramidase (ASAH1) protects retinal cells (ARPE19) from oxidative stress.J Lipid Res. 2019 Jan;60(1):30-43. doi: 10.1194/jlr.M082198. Epub 2018 Nov 9. J Lipid Res. 2019. PMID: 30413652 Free PMC article.
-
Ceramide Kinase Is Upregulated in Metastatic Breast Cancer Cells and Contributes to Migration and Invasion by Activation of PI 3-Kinase and Akt.Int J Mol Sci. 2020 Feb 19;21(4):1396. doi: 10.3390/ijms21041396. Int J Mol Sci. 2020. PMID: 32092937 Free PMC article.
-
Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process.Int J Mol Sci. 2024 Mar 28;25(7):3790. doi: 10.3390/ijms25073790. Int J Mol Sci. 2024. PMID: 38612601 Free PMC article. Review.
-
Skewing cPLA2α activity toward oxoeicosanoid production promotes neutrophil N2 polarization, wound healing, and the response to sepsis.Sci Signal. 2023 Jul 11;16(793):eadd6527. doi: 10.1126/scisignal.add6527. Epub 2023 Jul 11. Sci Signal. 2023. PMID: 37433004 Free PMC article.
References
-
- Goldberg S. R., Diegelmann R. F. 2010. Wound healing primer. Surg. Clin. North Am. 90: 1133–1146 - PubMed
-
- Berry D. P., Harding K. G., Stanton M. R., Jasani B., Ehrlich H. P. 1998. Human wound contraction: collagen organization, fibroblasts, and myofibroblasts. Plast. Reconstr. Surg. 102: 124–131, discussion 132–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

