Cell-to-cell transfer of Leishmania amazonensis amastigotes is mediated by immunomodulatory LAMP-rich parasitophorous extrusions
- PMID: 24824158
- PMCID: PMC4353215
- DOI: 10.1111/cmi.12311
Cell-to-cell transfer of Leishmania amazonensis amastigotes is mediated by immunomodulatory LAMP-rich parasitophorous extrusions
Abstract
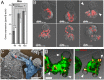
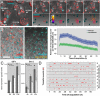
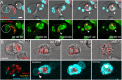
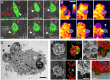
The last step of Leishmania intracellular life cycle is the egress of amastigotes from the host cell and their uptake by adjacent cells. Using multidimensional live imaging of long-term-infected macrophage cultures we observed that Leishmania amazonensis amastigotes were transferred from cell to cell when the donor host macrophage delivers warning signs of imminent apoptosis. They were extruded from the macrophage within zeiotic structures (membrane blebs, an apoptotic feature) rich in phagolysosomal membrane components. The extrusions containing amastigotes were selectively internalized by vicinal macrophages and the rescued amastigotes remain viable in recipient macrophages. Host cell apoptosis induced by micro-irradiation of infected macrophage nuclei promoted amastigotes extrusion, which were rescued by non-irradiated vicinal macrophages. Using amastigotes isolated from LAMP1/LAMP2 knockout fibroblasts, we observed that the presence of these lysosomal components on amastigotes increases interleukin 10 production. Enclosed within host cell membranes, amastigotes can be transferred from cell to cell without full exposure to the extracellular milieu, what represents an important strategy developed by the parasite to evade host immune system.
© 2014 The Authors. Cellular Microbiology published by John Wiley & Sons Ltd.
Figures
References
-
- Alvarez M. Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16:2161–2165. - PubMed
-
- Arruda DC, Santos LC, Melo FM, Pereira FV, Figueiredo CR, Matsuo AL, et al. beta-Actin-binding complementarity-determining region 2 of variable heavy chain from monoclonal antibody C7 induces apoptosis in several human tumor cells and is protective against metastatic melanoma. J Biol Chem. 2012;287:14912–14922. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

