Urinary metabolite profiles in premature infants show early postnatal metabolic adaptation and maturation
- PMID: 24824288
- PMCID: PMC4042575
- DOI: 10.3390/nu6051913
Urinary metabolite profiles in premature infants show early postnatal metabolic adaptation and maturation
Abstract
Objectives: Early nutrition influences metabolic programming and long-term health. We explored the urinary metabolite profiles of 48 premature infants (birth weight < 1500 g) randomized to an enhanced or a standard diet during neonatal hospitalization.
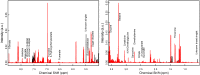
Methods: Metabolomics using nuclear magnetic resonance spectroscopy (NMR) was conducted on urine samples obtained during the first week of life and thereafter fortnightly.
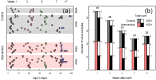
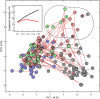
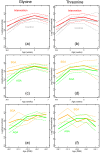
Results: The intervention group received significantly higher amounts of energy, protein, lipids, vitamin A, arachidonic acid and docosahexaenoic acid as compared to the control group. Enhanced nutrition did not appear to affect the urine profiles to an extent exceeding individual variation. However, in all infants the glucogenic amino acids glycine, threonine, hydroxyproline and tyrosine increased substantially during the early postnatal period, along with metabolites of the tricarboxylic acid cycle (succinate, oxoglutarate, fumarate and citrate). The metabolite changes correlated with postmenstrual age. Moreover, we observed elevated threonine and glycine levels in first-week urine samples of the small for gestational age (SGA; birth weight < 10th percentile for gestational age) as compared to the appropriate for gestational age infants.
Conclusion: This first nutri-metabolomics study in premature infants demonstrates that the physiological adaptation during the fetal-postnatal transition as well as maturation influences metabolism during the breastfeeding period. Elevated glycine and threonine levels were found in the first week urine samples of the SGA infants and emerged as potential biomarkers of an altered metabolic phenotype.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

