Unveiling novel interactions of histone chaperone Asf1 linked to TREX-2 factors Sus1 and Thp1
- PMID: 24824343
- PMCID: PMC4133220
- DOI: 10.4161/nucl.29155
Unveiling novel interactions of histone chaperone Asf1 linked to TREX-2 factors Sus1 and Thp1
Abstract
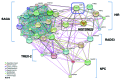
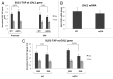
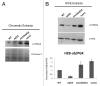
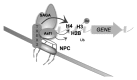
Anti-silencing function 1 (Asf1) is a conserved key eukaryotic histone H3/H4 chaperone that participates in a variety of DNA and chromatin-related processes. These include the assembly and disassembly of histones H3 and H4 from chromatin during replication, transcription, and DNA repair. In addition, Asf1 is required for H3K56 acetylation activity dependent on histone acetyltransferase Rtt109. Thus, Asf1 impacts on many aspects of DNA metabolism. To gain insights into the functional links of Asf1 with other cellular machineries, we employed mass spectrometry coupled to tandem affinity purification (TAP) to investigate novel physical interactions of Asf1. Under different TAP-MS analysis conditions, we describe a new repertoire of Asf1 physical interactions and novel Asf1 post-translational modifications as ubiquitination, methylation and acetylation that open up new ways to regulate Asf1 functions. Asf1 co-purifies with several subunits of the TREX-2, SAGA complexes, and with nucleoporins Nup2, Nup60, and Nup57, which are all involved in transcription coupled to mRNA export in eukaryotes. Reciprocally, Thp1 and Sus1 interact with Asf1. Albeit mRNA export and GAL1 transcription are not affected in asf1Δ a strong genetic interaction exists between ASF1 and SUS1. Notably, supporting a functional link between Asf1 and TREX-2, both Sus1 and Thp1 affect the levels of Asf1-dependent histone H3K56 acetylation and histone H3 and H4 incorporation onto chromatin. Additionally, we provide evidence for a role of Asf1 in histone H2B ubiquitination. This work proposes a functional link between Asf1 and TREX-2 components in histone metabolism at the vicinity of the nuclear pore complex.
Keywords: (5-10) yAsf1; SAGA; Sus1; TAP-MS strategy; TREX-2; histones; yeast.
Figures



Similar articles
-
Mutational uncoupling of the role of Sus1 in nuclear pore complex targeting of an mRNA export complex and histone H2B deubiquitination.J Biol Chem. 2009 May 1;284(18):12049-56. doi: 10.1074/jbc.M900502200. Epub 2009 Mar 5. J Biol Chem. 2009. PMID: 19269973 Free PMC article.
-
Two factor authentication: Asf1 mediates crosstalk between H3 K14 and K56 acetylation.Nucleic Acids Res. 2019 Aug 22;47(14):7380-7391. doi: 10.1093/nar/gkz508. Nucleic Acids Res. 2019. PMID: 31194870 Free PMC article.
-
Utilizing targeted mass spectrometry to demonstrate Asf1-dependent increases in residue specificity for Rtt109-Vps75 mediated histone acetylation.PLoS One. 2015 Mar 17;10(3):e0118516. doi: 10.1371/journal.pone.0118516. eCollection 2015. PLoS One. 2015. PMID: 25781956 Free PMC article.
-
The histone chaperone Asf1 at the crossroads of chromatin and DNA checkpoint pathways.Chromosoma. 2007 Apr;116(2):79-93. doi: 10.1007/s00412-006-0087-z. Epub 2006 Dec 19. Chromosoma. 2007. PMID: 17180700 Review.
-
Sus1/ENY2: a multitasking protein in eukaryotic gene expression.Crit Rev Biochem Mol Biol. 2012 Nov-Dec;47(6):556-68. doi: 10.3109/10409238.2012.730498. Epub 2012 Oct 12. Crit Rev Biochem Mol Biol. 2012. PMID: 23057668 Review.
Cited by
-
Comprehensive analysis of interacting proteins and genome-wide location studies of the Sas3-dependent NuA3 histone acetyltransferase complex.FEBS Open Bio. 2014 Nov 8;4:996-1006. doi: 10.1016/j.fob.2014.11.001. eCollection 2014. FEBS Open Bio. 2014. PMID: 25473596 Free PMC article.
-
A role for Mog1 in H2Bub1 and H3K4me3 regulation affecting RNAPII transcription and mRNA export.EMBO Rep. 2018 Nov;19(11):e45992. doi: 10.15252/embr.201845992. Epub 2018 Sep 24. EMBO Rep. 2018. PMID: 30249596 Free PMC article.
-
Data for the identification of proteins and post-translational modifications of proteins associated to histones H3 and H4 in S. cerevisiae, using tandem affinity purification coupled with mass spectrometry.Data Brief. 2016 Feb 5;6:965-9. doi: 10.1016/j.dib.2016.01.068. eCollection 2016 Mar. Data Brief. 2016. PMID: 26949727 Free PMC article.
-
The evolutionarily conserved factor Sus1/ENY2 plays a role in telomere length maintenance.Curr Genet. 2018 Jun;64(3):635-644. doi: 10.1007/s00294-017-0778-4. Epub 2017 Nov 7. Curr Genet. 2018. PMID: 29116388
-
Translation of Genotype to Phenotype by a Hierarchy of Cell Subsystems.Cell Syst. 2016 Feb 24;2(2):77-88. doi: 10.1016/j.cels.2016.02.003. Cell Syst. 2016. PMID: 26949740 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
