Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node
- PMID: 24824450
- PMCID: PMC4091760
- DOI: 10.1016/j.cbi.2014.05.001
Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node
Abstract
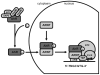
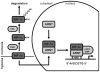
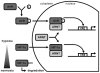
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that mediates many of the responses to toxic environmental chemicals such as TCDD or dioxin-like PCBs. To regulate gene expression, the AhR requires its binding partner, the aryl hydrocarbon receptor nuclear translocator (ARNT). ARNT is also required by the hypoxia-inducible factor-1α (HIF-1α), a crucial regulator of responses to conditions of reduced oxygen. The important role of ARNT in both the AhR and HIF-1α signaling pathways establishes a meaningful foundation for a possible crosstalk between these two vitally important signaling pathways. This crosstalk might lead to interference between the two signaling pathways and thus might play a role in the variety of cellular responses after exposure to AhR ligands and reduced oxygen availability. This review focuses on studies that have analyzed the effect of low oxygen environments and hypoxia-mimetic agents on AhR signaling and conversely, the effect of AhR ligands, with a special emphasis on PCBs, on HIF-1α signaling. We highlight studies that assess the role of ARNT, elucidate the mechanism of the crosstalk, and discuss the physiological implications for exposure to AhR-inducing compounds in the context of hypoxia.
Keywords: ARNT; Crosstalk; Environmental toxicants; Metabolism; Oxygen sensing.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Figures
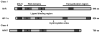
Similar articles
-
Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines.Toxicol Appl Pharmacol. 2014 Feb 1;274(3):408-16. doi: 10.1016/j.taap.2013.12.002. Epub 2013 Dec 16. Toxicol Appl Pharmacol. 2014. PMID: 24355420 Free PMC article.
-
NcoA2-Dependent Inhibition of HIF-1α Activation Is Regulated via AhR.Toxicol Sci. 2015 Dec;148(2):517-30. doi: 10.1093/toxsci/kfv199. Epub 2015 Sep 8. Toxicol Sci. 2015. PMID: 26350169
-
Hypoxia inhibits induction of aryl hydrocarbon receptor activity in topminnow hepatocarcinoma cells in an ARNT-dependent manner.Comp Biochem Physiol C Toxicol Pharmacol. 2009 Sep;150(3):383-9. doi: 10.1016/j.cbpc.2009.06.003. Epub 2009 Jun 16. Comp Biochem Physiol C Toxicol Pharmacol. 2009. PMID: 19539049 Free PMC article.
-
AhR and ARNT modulate ER signaling.Toxicology. 2010 Feb 9;268(3):132-8. doi: 10.1016/j.tox.2009.09.007. Epub 2009 Sep 22. Toxicology. 2010. PMID: 19778576 Review.
-
Hypoxia-inducible aryl hydrocarbon receptor nuclear translocator (ARNT) (HIF-1β): is it a rare exception?Mol Med. 2014 May 27;20(1):215-20. doi: 10.2119/molmed.2014.00032. Mol Med. 2014. PMID: 24849811 Free PMC article. Review.
Cited by
-
Indoleamine 2,3 Dioxygenase 1-The Potential Link between the Innate Immunity and the Ischemia-Reperfusion-Induced Acute Kidney Injury?Int J Mol Sci. 2022 May 31;23(11):6176. doi: 10.3390/ijms23116176. Int J Mol Sci. 2022. PMID: 35682852 Free PMC article. Review.
-
CncC/Maf-mediated xenobiotic response pathway in insects.Arch Insect Biochem Physiol. 2020 Jun;104(2):e21674. doi: 10.1002/arch.21674. Epub 2020 Apr 12. Arch Insect Biochem Physiol. 2020. PMID: 32281173 Free PMC article. Review.
-
Transcobalamin 2 orchestrates monocyte proliferation and TLR4-driven inflammation in systemic lupus erythematosus via folate one-carbon metabolism.Front Immunol. 2024 May 31;15:1339680. doi: 10.3389/fimmu.2024.1339680. eCollection 2024. Front Immunol. 2024. PMID: 38881906 Free PMC article.
-
Aryl hydrocarbon receptor (AhR) reveals evidence of antagonistic pleiotropy in the regulation of the aging process.Cell Mol Life Sci. 2022 Aug 20;79(9):489. doi: 10.1007/s00018-022-04520-x. Cell Mol Life Sci. 2022. PMID: 35987825 Free PMC article. Review.
-
Genome-wide scan reveals signatures of selection related to pollution adaptation in non-model estuarine Atlantic killifish (Fundulus heteroclitus).Aquat Toxicol. 2018 Jul;200:73-82. doi: 10.1016/j.aquatox.2018.04.017. Epub 2018 Apr 25. Aquat Toxicol. 2018. PMID: 29727773 Free PMC article.
References
-
- Allen JW, Johnson RS, Bhatia SN. Hypoxic inhibition of 3-methylcholanthrene-induced CYP1A1 expression is independent of HIF-1alpha. Toxicol Lett. 2005;155:151–159. - PubMed
-
- Bacsi SG, Reisz-Porszasz S, Hankinson O. Orientation of the heterodimeric aryl hydrocarbon (dioxin) receptor complex on its asymmetric DNA recognition sequence. Mol Pharmacol. 1995;47:432–438. - PubMed
-
- Bieche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics. 2007;17:731–742. - PubMed
-
- Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J Biol Chem. 1999;274:12115–12123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

