Mucosal immunization with integrase-defective lentiviral vectors protects against influenza virus challenge in mice
- PMID: 24824623
- PMCID: PMC4019533
- DOI: 10.1371/journal.pone.0097270
Mucosal immunization with integrase-defective lentiviral vectors protects against influenza virus challenge in mice
Abstract
Recent reports highlight the potential for integrase-defective lentiviral vectors (IDLV) to be developed as vaccines due to their ability to elicit cell-mediated and humoral immune responses after intramuscular administration. Differently from their integrase-competent counterpart, whose utility for vaccine development is limited by the potential for insertional mutagenesis, IDLV possess a mutation in their integrase gene that prevents genomic integration. Instead, they are maintained as episomal DNA circles that retain the ability to stably express functional proteins. Despite their favorable profile, it is unknown whether IDLV elicit immune responses after intranasal administration, a route that could be advantageous in the case of infection with a respiratory agent. Using influenza as a model, we constructed IDLV expressing the influenza virus nucleoprotein (IDLV-NP), and tested their ability to generate NP-specific immune responses and protect from challenge in vivo. We found that administration of IDLV-NP elicited NP-specific T cell and antibody responses in BALB/c mice. Importantly, IDLV-NP was protective against homologous and heterosubtypic influenza virus challenge only when given by the intranasal route. This is the first report demonstrating that IDLV can induce protective immunity after intranasal administration, and suggests that IDLV may represent a promising vaccine platform against infectious agents.
Conflict of interest statement
Figures




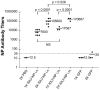

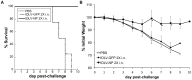
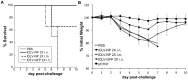
References
-
- Brave A, Ljungberg K, Wahren B, Liu MA (2007) Vaccine delivery methods using viral vectors. Mol Pharm 4: 18–32. - PubMed
-
- Buffa V, Negri DR, Leone P, Bona R, Borghi M, et al. (2006) A single administration of lentiviral vectors expressing either full-length human immunodeficiency virus 1 (HIV-1)(HXB2) Rev/Env or codon-optimized HIV-1(JR-FL) gp120 generates durable immune responses in mice. The Journal of general virology 87: 1625–1634. - PubMed
-
- Iglesias MC, Frenkiel MP, Mollier K, Souque P, Despres P, et al. (2006) A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. The journal of gene medicine 8: 265–274. - PubMed
-
- He Y, Zhang J, Mi Z, Robbins P, Falo LD Jr (2005) Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. Journal of immunology 174: 3808–3817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

