Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans
- PMID: 24824668
- PMCID: PMC4992082
- DOI: 10.1038/ismej.2014.73
Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans
Abstract
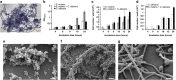
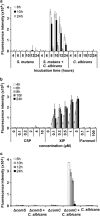
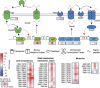
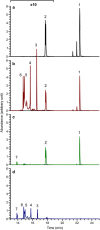
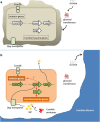
Polymicrobial biofilms are of large medical importance, but relatively little is known about the role of interspecies interactions for their physiology and virulence. Here, we studied two human pathogens co-occuring in the oral cavity, the opportunistic fungus Candida albicans and the caries-promoting bacterium Streptococcus mutans. Dual-species biofilms reached higher biomass and cell numbers than mono-species biofilms, and the production of extracellular polymeric substances (EPSs) by S. mutans was strongly suppressed, which was confirmed by scanning electron microscopy, gas chromatography-mass spectrometry and transcriptome analysis. To detect interkingdom communication, C. albicans was co-cultivated with a strain of S. mutans carrying a transcriptional fusion between a green fluorescent protein-encoding gene and the promoter for sigX, the alternative sigma factor of S. mutans, which is induced by quorum sensing signals. Strong induction of sigX was observed in dual-species biofilms, but not in single-species biofilms. Conditioned media from mixed biofilms but not from C. albicans or S. mutans cultivated alone activated sigX in the reporter strain. Deletion of comS encoding the synthesis of the sigX-inducing peptide precursor abolished this activity, whereas deletion of comC encoding the competence-stimulating peptide precursor had no effect. Transcriptome analysis of S. mutans confirmed induction of comS, sigX, bacteriocins and the downstream late competence genes, including fratricins, in dual-species biofilms. We show here for the first time the stimulation of the complete quorum sensing system of S. mutans by a species from another kingdom, namely the fungus C. albicans, resulting in fundamentally changed virulence properties of the caries pathogen.
Figures





Similar articles
-
Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans.Microbiologyopen. 2019 Dec;8(12):e937. doi: 10.1002/mbo3.937. Epub 2019 Sep 27. Microbiologyopen. 2019. PMID: 31560838 Free PMC article.
-
The Alternative Sigma Factor SigX Controls Bacteriocin Synthesis and Competence, the Two Quorum Sensing Regulated Traits in Streptococcus mutans.PLoS Genet. 2015 Jul 9;11(7):e1005353. doi: 10.1371/journal.pgen.1005353. eCollection 2015 Jul. PLoS Genet. 2015. PMID: 26158727 Free PMC article.
-
Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation.Eukaryot Cell. 2009 Nov;8(11):1658-64. doi: 10.1128/EC.00070-09. Epub 2009 Aug 28. Eukaryot Cell. 2009. PMID: 19717744 Free PMC article.
-
Multifaceted roles of Candida albicans and Streptococcus mutans in contributing to polybiofilm infections in early childhood caries.Front Cell Infect Microbiol. 2025 Jun 27;15:1625103. doi: 10.3389/fcimb.2025.1625103. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40654570 Free PMC article. Review.
-
Quorum sensing and biofilm formation by Streptococcus mutans.Adv Exp Med Biol. 2008;631:178-88. doi: 10.1007/978-0-387-78885-2_12. Adv Exp Med Biol. 2008. PMID: 18792689 Review.
Cited by
-
Lactic acid bacteria differentially regulate filamentation in two heritable cell types of the human fungal pathogen Candida albicans.Mol Microbiol. 2016 Nov;102(3):506-519. doi: 10.1111/mmi.13475. Epub 2016 Aug 18. Mol Microbiol. 2016. PMID: 27479705 Free PMC article.
-
The ultrastructure of subgingival dental plaque, revealed by high-resolution field emission scanning electron microscopy.BDJ Open. 2015 Nov 27;1:15003. doi: 10.1038/bdjopen.2015.3. eCollection 2015. BDJ Open. 2015. PMID: 29607057 Free PMC article.
-
Comparing the cariogenic species Streptococcus sobrinus and S. mutans on whole genome level.J Oral Microbiol. 2014 Dec 3;6:26189. doi: 10.3402/jom.v6.26189. eCollection 2014. J Oral Microbiol. 2014. PMID: 25475081 Free PMC article.
-
Streptococcus gordonii comCDE (competence) operon modulates biofilm formation with Candida albicans.Microbiology (Reading). 2015 Feb;161(Pt 2):411-421. doi: 10.1099/mic.0.000010. Epub 2014 Dec 10. Microbiology (Reading). 2015. PMID: 25505189 Free PMC article.
-
Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans.Microbiologyopen. 2019 Dec;8(12):e937. doi: 10.1002/mbo3.937. Epub 2019 Sep 27. Microbiologyopen. 2019. PMID: 31560838 Free PMC article.
References
-
- Bandara HM, K Cheung BP, Watt RM, Jin LJ, Samaranayake LP. (2013). Pseudomonas aeruginosa lipopolysaccharide inhibits Candida albicans hyphae formation and alters gene expression during biofilm development. Mol Oral Microbiol 28: 54–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

