Structure-activity relationship of benzophenanthridine alkaloids from Zanthoxylum rhoifolium having antimicrobial activity
- PMID: 24824737
- PMCID: PMC4019524
- DOI: 10.1371/journal.pone.0097000
Structure-activity relationship of benzophenanthridine alkaloids from Zanthoxylum rhoifolium having antimicrobial activity
Abstract
Zanthoxylum rhoifolium (Rutaceae) is a plant alkaloid that grows in South America and has been used in Brazilian traditional medicine for the treatment of different health problems. The present study was designed to evaluate the antimicrobial activity of the steam bark crude methanol extract, fractions, and pure alkaloids of Z. rhoifolium. Its stem bark extracts exhibited a broad spectrum of antimicrobial activity, ranging from 12.5 to 100 µg/mL using bioautography method, and from 125 to 500 µg/mL in the microdilution bioassay. From the dichloromethane basic fraction, three furoquinoline alkaloids (1-3), and nine benzophenanthridine alkaloids (4-12) were isolated and the antimicrobial activity of the benzophenanthridine alkaloids is discussed in terms of structure-activity relationships. The alkaloid with the widest spectrum of activity was chelerythrine (10), followed by avicine (12) and dihydrochelerythrine (4). The minimal inhibitory concentrations of chelerythrine, of 1.50 µg/mL for all bacteria tested, and between 3.12 and 6.25 µg/mL for the yeast tested, show this compound to be a more powerful antimicrobial agent when compared with the other active alkaloids isolated from Z. rhoifolium. To verify the potential importance of the methylenedioxy group (ring A) of these alkaloids, chelerythrine was selected to represent the remainder of the benzophenanthridine alkaloids isolated in this work and was subjected to a demethylation reaction giving derivative 14. Compared to chelerythrine, the derivative (14) was less active against the tested bacteria and fungi. Kinetic measurements of the bacteriolytic activities of chelerythrine against the bacteria Bacillus subtilis (Gram-positive) and Escherichia coli (Gram-negative) were determined by optical density based on real time assay, suggesting that its mechanism of action is not bacteriolytic. The present study did not detect hemolytic effects of chelerythrine on erythrocytes and found a protective effect considering the decrease in TBARS and AOPP (advanced oxidized protein products) levels when compared to the control group.
Conflict of interest statement
Figures


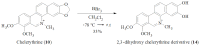
Similar articles
-
Interaction of the putative anticancer alkaloid chelerythrine with nucleic acids: biophysical perspectives.Biophys Rev. 2020 Oct 31;12(6):1369-86. doi: 10.1007/s12551-020-00769-3. Online ahead of print. Biophys Rev. 2020. PMID: 33131000 Free PMC article. Review.
-
Cytotoxicity and Genotoxicity Evaluation of Zanthoxylum rhoifolium Lam and In Silico Studies of Its Alkaloids.Molecules. 2023 Jul 11;28(14):5336. doi: 10.3390/molecules28145336. Molecules. 2023. PMID: 37513210 Free PMC article.
-
Validation of use of a traditional antimalarial remedy from French Guiana, Zanthoxylum rhoifolium Lam.J Ethnopharmacol. 2006 Jul 19;106(3):348-52. doi: 10.1016/j.jep.2006.01.011. Epub 2006 Feb 28. J Ethnopharmacol. 2006. PMID: 16504432
-
Antibacterial alkaloids from Zanthoxylum rhoifolium.Planta Med. 2003 Apr;69(4):371-4. doi: 10.1055/s-2003-38882. Planta Med. 2003. PMID: 12709908
-
Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities.Molecules. 2019 Dec 3;24(23):4419. doi: 10.3390/molecules24234419. Molecules. 2019. PMID: 31816948 Free PMC article. Review.
Cited by
-
In Vitro Antimicrobial and Antiproliferative Activities of the Root Bark Extract and Isolated Chemical Constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae).Plants (Basel). 2020 Jul 21;9(7):920. doi: 10.3390/plants9070920. Plants (Basel). 2020. PMID: 32708115 Free PMC article.
-
Interaction of the putative anticancer alkaloid chelerythrine with nucleic acids: biophysical perspectives.Biophys Rev. 2020 Oct 31;12(6):1369-86. doi: 10.1007/s12551-020-00769-3. Online ahead of print. Biophys Rev. 2020. PMID: 33131000 Free PMC article. Review.
-
Phytochemical Analysis and Antimicrobial Efficacy of Macleaya cordata against Extensively Drug-Resistant Staphylococcus aureus.Nat Prod Commun. 2018 Nov;13(11):10.1177/1934578X1801301117. doi: 10.1177/1934578X1801301117. Epub 2018 Nov 1. Nat Prod Commun. 2018. PMID: 31080542 Free PMC article.
-
Can Nature Overcome Invasive Gastrointestinal Infections?Int J Mol Sci. 2025 Jun 17;26(12):5795. doi: 10.3390/ijms26125795. Int J Mol Sci. 2025. PMID: 40565258 Free PMC article. Review.
-
Cytotoxicity and Genotoxicity Evaluation of Zanthoxylum rhoifolium Lam and In Silico Studies of Its Alkaloids.Molecules. 2023 Jul 11;28(14):5336. doi: 10.3390/molecules28145336. Molecules. 2023. PMID: 37513210 Free PMC article.
References
-
- Taylor PW (2013) Alternative natural sources for a new generation of antibacterial agents. Int J Antimicrob Agents 42: 195–201. - PubMed
-
- Tin-Wa M, Bell CL, Bevelle C, Fong HHS, Farnsworth NR (1974) Potencial anticancer agents I: Confirming evidence for the structure of fagaronine. J Pharm Sci 63: 1476–77. - PubMed
-
- Pereira SS, Lopes LS, Marques RB, Figueiredo KA, Costa DA, et al. (2010) Antinociceptive effect of Zanthoxylum rhoifolium Lam. (Rutaceae) in models of acute pain in rodents. J Ethnopharmacol 129: 227–31. - PubMed
-
- Freitas FFBP, Fernandes HB, Piauilino CA, Pereira SS, Carvalho KIM, et al. (2011) Gastroprotective activity of Zanthoxylum rhoifolium Lam. In animal model. J Ethnopharmacol 137: 00–8. - PubMed
-
- Jullian V, Bourdy G, Georges S, Maurel S, Sauvain M (2006) Validation of use of a traditional antimalarial remedy from French Guiana, Zanthoxilum rhoifolium Lam. J Ethnopharmacol 106: 348–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

