Functional production of a soluble and secreted single-chain antibody by a bacterial secretion system
- PMID: 24824752
- PMCID: PMC4019604
- DOI: 10.1371/journal.pone.0097367
Functional production of a soluble and secreted single-chain antibody by a bacterial secretion system
Abstract
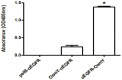
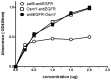
Single-chain variable fragments (scFvs) serve as an alternative to full-length monoclonal antibodies used in research and therapeutic and diagnostic applications. However, when recombinant scFvs are overexpressed in bacteria, they often form inclusion bodies and exhibit loss of function. To overcome this problem, we developed an scFv secretion system in which scFv was fused with osmotically inducible protein Y (osmY), a bacterial secretory carrier protein, for efficient protein secretion. Anti-EGFR scFv (αEGFR) was fused with osmY (N- and C-termini) and periplasmic leader sequence (pelB) to generate αEGFR-osmY, osmY-αEGFR, and pelB-αEGFR (control), respectively. In comparison with the control, both the osmY-fused αEGFR scFvs were soluble and secreted into the LB medium. Furthermore, the yield of soluble αEGFR-osmY was 20-fold higher, and the amount of secreted protein was 250-fold higher than that of osmY-αEGFR. In addition, the antigen-binding activity of both the osmY-fused αEGFRs was 2-fold higher than that of the refolded pelB-αEGFR from inclusion bodies. Similar results were observed with αTAG72-osmY and αHer2-osmY. These results suggest that the N-terminus of osmY fused with scFv produces a high yield of soluble, functional, and secreted scFv, and the osmY-based bacterial secretion system may be used for the large-scale industrial production of low-cost αEGFR protein.
Conflict of interest statement
Figures


References
-
- Lorberboum-Galski H (2011) Human toxin-based recombinant immunotoxins/chimeric proteins as a drug delivery system for targeted treatment of human diseases. Expert Opin Drug Deliv 8: 605–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

