Forecasting cell death dose-response from early signal transduction responses in vitro
- PMID: 24824809
- PMCID: PMC4176052
- DOI: 10.1093/toxsci/kfu089
Forecasting cell death dose-response from early signal transduction responses in vitro
Abstract
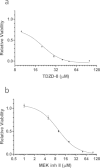
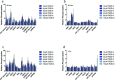
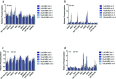
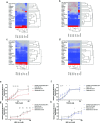
The rapid pharmacodynamic response of cells to toxic xenobiotics is primarily coordinated by signal transduction networks, which follow a simple framework: the phosphorylation/dephosphorylation cycle mediated by kinases and phosphatases. However, the time course from initial pharmacodynamic response(s) to cell death following exposure can have a vast range. Viewing this time lag between early signaling events and the ultimate cellular response as an opportunity, we hypothesize that monitoring the phosphorylation of proteins related to cell death and survival pathways at key, early time points may be used to forecast a cell's eventual fate, provided that we can measure and accurately interpret the protein responses. In this paper, we focused on a three-phased approach to forecast cell death after exposure: (1) determine time points relevant to important signaling events (protein phosphorylation) by using estimations of adenosine triphosphate production to reflect the relationship between mitochondrial-driven energy metabolism and kinase response, (2) experimentally determine phosphorylation values for proteins related to cell death and/or survival pathways at these significant time points, and (3) use cluster analysis to predict the dose-response relationship between cellular exposure to a xenobiotic and plasma membrane degradation at 24 h post-exposure. To test this approach, we exposed HepG2 cells to two disparate treatments: a GSK-3β inhibitor and a MEK inhibitor. After using our three-phased approach, we were able to accurately forecast the 24 h HepG2 plasma membrane degradation dose-response from protein phosphorylation values as early as 20 min post-MEK inhibitor exposure and 40 min post-GSK-3β exposure.
Keywords: cytotoxicity; dose-response; kinase; predictive toxicology; signal transduction.
© The Author 2014. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Ainscow E. K., Brand M. D. Top-down control analysis of ATP turnover, glycolysis and oxidative phosphorylation in rat hepatocytes. Eur. J. Biochem. 1999;263:671–685. - PubMed
-
- Anjum R., Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat. Rev. Mol. Cell. Biol. 2008;9:747–758. - PubMed
-
- Bakare O., Ashendel C. L., Peng H., Zalkow L. H., Burgess E. M. Synthesis and MEK1 inhibitory activities of imido-substituted 2-chloro-1,4-naphthoquinones. Bioorg. Med. Chem. 2003;11:3165–3170. - PubMed
-
- Ballif B. A., Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) Cell. Growth Differ. 2001;12:397–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

