Recurrent MYOD1 mutations in pediatric and adult sclerosing and spindle cell rhabdomyosarcomas: evidence for a common pathogenesis
- PMID: 24824843
- PMCID: PMC4108340
- DOI: 10.1002/gcc.22187
Recurrent MYOD1 mutations in pediatric and adult sclerosing and spindle cell rhabdomyosarcomas: evidence for a common pathogenesis
Abstract
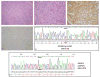
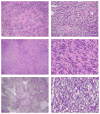
Sclerosing and spindle cell rhabdomyosarcoma (RMS) are rare types of RMS recently reclassified as a stand-alone pathologic entity, separate from embryonal RMS (ERMS). Although sclerosing and spindle cell RMS share clinical and morphologic features, a pathogenetic link based on shared molecular alterations has not been established. Spindle cell RMS in children have been associated with a less aggressive clinical course compared to adults. Recently, recurrent MYOD1 mutations were described in 44% of adult spindle cell RMS, but no pediatric tumors or sclerosing RMS were studied for comparison. Thus, we investigated 16 RMS (5 sclerosing and 11 spindle cell) in children and adults for the presence of MYOD1 mutations by targeted Polymerase Chain Reaction (PCR). Remarkably, all 5 sclerosing RMS and 4 of 11 spindle cell RMS showed the MYOD1 p.L122R hot-spot mutation. Of the five pediatric tumors, 2/2 sclerosing RMS and 2/3 spindle cell RMS showed MYOD1 mutations. Three of nine MYOD1-mutant RMS showed coexistent PIK3CA mutations, while no MDM2 amplifications were identified. All four pediatric MYOD1-mutated RMS patients died of the disease at 12-35 months following diagnosis. In conclusion, spindle cell and sclerosing RMS show recurrent MYOD1 mutations, in keeping with a single pathologic entity, regardless of age at presentation. This group however, is distinct from the infantile RMS associated with NCOA2 fusions. Although our study suggests that pediatric MYOD1-mutant RMS follow an aggressive behavior with high mortality, further studies are required to confirm this finding.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
Figures

References
-
- Antonescu CR, Sommer G, Sarran L, Tschernyavsky SJ, Riedel E, Woodruff JM, Robson M, Maki R, Brennan MF, Ladanyi M, DeMatteo RP, Besmer P. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003;9(9):3329–3337. - PubMed
-
- Bouron-Dal Soglio D, Rougemont AL, Absi R, Barrette S, Montpetit A, Fetni R, Fournet JC. SNP genotyping of a sclerosing rhabdomyosarcoma: reveals highly aneuploid profile and a specific MDM2/HMGA2 amplification. Hum Pathol. 2009;40(9):1347–1352. - PubMed
-
- Cavazzana AO, Schmidt D, Ninfo V, Harms D, Tollot M, Carli M, Treuner J, Betto R, Salviati G. Spindle cell rhabdomyosarcoma. A prognostically favorable variant of rhabdomyosarcoma. Am J Surg Pathol. 1992;16(3):229–235. - PubMed
-
- Chiles MC, Parham DM, Qualman SJ, Teot LA, Bridge JA, Ullrich F, Barr FG, Meyer WH Soft Tissue Sarcoma Committee of the Children’s Oncology G. Sclerosing rhabdomyosarcomas in children and adolescents: a clinicopathologic review of 13 cases from the Intergroup Rhabdomyosarcoma Study Group and Children’s Oncology Group. Pediatr Dev Pathol. 2004;7(6):583–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

