Angiotensin-(1-7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway
- PMID: 24824997
- PMCID: PMC4241089
- DOI: 10.1111/bph.12770
Angiotensin-(1-7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway
Abstract
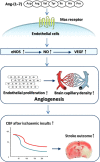
Background and purpose: As a newer component of the renin-angiotensin system, angiotensin-(1-7) [Ang-(1-7) ] has been shown to facilitate angiogenesis and protect against ischaemic damage in peripheral tissues. However, the role of Ang-(1-7) in brain angiogenesis remains unclear. The aim of this study was to investigate whether Ang-(1-7) could promote angiogenesis in brain, thus inducing tolerance against focal cerebral ischaemia.
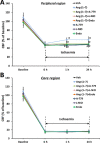
Experimental approach: Male Sprague-Dawley rats were i.c.v. infused with Ang-(1-7), A-779 (a Mas receptor antagonist), L-NIO, a specific endothelial NOS (eNOS) inhibitor, endostatin (an anti-angiogenic compound) or vehicle, alone or simultaneously, for 1-4 weeks. Capillary density, endothelial cell proliferation and key components of eNOS pathway in the brain were evaluated. Afterwards, rats were subjected to permanent middle cerebral artery occlusion (pMCAO), and regional cerebral blood flow (rCBF), infarct volume and neurological deficits were measured 24 h later.
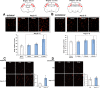
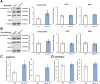
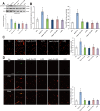
Key results: Infusion of Ang-(1-7) for 4 weeks significantly increased brain capillary density via promoting endothelial cell proliferation, which was accompanied by eNOS activation and up-regulation of NO and VEGF in brain. These effects were abolished by A-779 or L-NIO. More importantly, Ang-(1-7) improved rCBF and decreased infarct volume and neurological deficits after pMCAO, which could be reversed by A-779, L-NIO or endostatin.
Conclusions and implications: This is the first evidence that Ang-(1-7) promotes brain angiogenesis via a Mas/eNOS-dependent pathway, which enhances tolerance against subsequent cerebral ischaemia. These findings highlight brain Ang-(1-7)/Mas signalling as a potential target in stroke prevention.
© 2014 The British Pharmacological Society.
Figures

Similar articles
-
Suppressing inflammation by inhibiting the NF-κB pathway contributes to the neuroprotective effect of angiotensin-(1-7) in rats with permanent cerebral ischaemia.Br J Pharmacol. 2012 Dec;167(7):1520-32. doi: 10.1111/j.1476-5381.2012.02105.x. Br J Pharmacol. 2012. PMID: 22817481 Free PMC article.
-
Cerebroprotection by angiotensin-(1-7) in endothelin-1-induced ischaemic stroke.Exp Physiol. 2011 Oct;96(10):1084-96. doi: 10.1113/expphysiol.2011.058578. Epub 2011 Jun 17. Exp Physiol. 2011. PMID: 21685445 Free PMC article.
-
Neuroprotection by post-stroke administration of an oral formulation of angiotensin-(1-7) in ischaemic stroke.Exp Physiol. 2018 Jun;103(6):916-923. doi: 10.1113/EP086957. Exp Physiol. 2018. PMID: 29663576
-
Neuronal over-expression of ACE2 protects brain from ischemia-induced damage.Neuropharmacology. 2014 Apr;79:550-8. doi: 10.1016/j.neuropharm.2014.01.004. Epub 2014 Jan 15. Neuropharmacology. 2014. PMID: 24440367 Free PMC article.
-
Angiotensin-(1-7): beyond the cardio-renal actions.Clin Sci (Lond). 2013 Apr;124(7):443-56. doi: 10.1042/CS20120461. Clin Sci (Lond). 2013. PMID: 23249272 Review.
Cited by
-
The counter regulatory axis of the renin angiotensin system in the brain and ischaemic stroke: Insight from preclinical stroke studies and therapeutic potential.Cell Signal. 2020 Dec;76:109809. doi: 10.1016/j.cellsig.2020.109809. Epub 2020 Oct 13. Cell Signal. 2020. PMID: 33059037 Free PMC article. Review.
-
The Renin Angiotensin System as a Therapeutic Target in Traumatic Brain Injury.Neurotherapeutics. 2023 Oct;20(6):1565-1591. doi: 10.1007/s13311-023-01435-8. Epub 2023 Sep 27. Neurotherapeutics. 2023. PMID: 37759139 Free PMC article. Review.
-
SARS-CoV-2 involvement in central nervous system tissue damage.Neural Regen Res. 2022 Jun;17(6):1228-1239. doi: 10.4103/1673-5374.327323. Neural Regen Res. 2022. PMID: 34782556 Free PMC article. Review.
-
Cognitive benefits of angiotensin IV and angiotensin-(1-7): A systematic review of experimental studies.Neurosci Biobehav Rev. 2018 Sep;92:209-225. doi: 10.1016/j.neubiorev.2018.05.005. Epub 2018 May 4. Neurosci Biobehav Rev. 2018. PMID: 29733881 Free PMC article.
-
Angiotensin (1-7) Expressing Probiotic as a Potential Treatment for Dementia.Front Aging. 2021 Mar;2:629164. doi: 10.3389/fragi.2021.629164. Epub 2021 Mar 30. Front Aging. 2021. PMID: 34901930 Free PMC article.
References
-
- Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, et al. Socioeconomic status and stroke: an updated review. Stroke. 2012;43:1186–1191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

