Coordinating bacterial cell division with nutrient availability: a role for glycolysis
- PMID: 24825009
- PMCID: PMC4030479
- DOI: 10.1128/mBio.00935-14
Coordinating bacterial cell division with nutrient availability: a role for glycolysis
Abstract
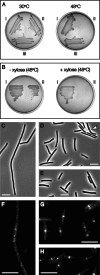
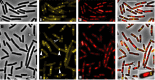
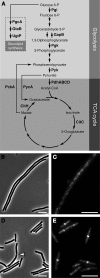
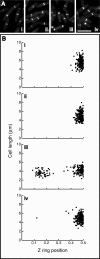
Cell division in bacteria is driven by a cytoskeletal ring structure, the Z ring, composed of polymers of the tubulin-like protein FtsZ. Z-ring formation must be tightly regulated to ensure faithful cell division, and several mechanisms that influence the positioning and timing of Z-ring assembly have been described. Another important but as yet poorly understood aspect of cell division regulation is the need to coordinate division with cell growth and nutrient availability. In this study, we demonstrated for the first time that cell division is intimately linked to central carbon metabolism in the model Gram-positive bacterium Bacillus subtilis. We showed that a deletion of the gene encoding pyruvate kinase (pyk), which produces pyruvate in the final reaction of glycolysis, rescues the assembly defect of a temperature-sensitive ftsZ mutant and has significant effects on Z-ring formation in wild-type B. subtilis cells. Addition of exogenous pyruvate restores normal division in the absence of the pyruvate kinase enzyme, implicating pyruvate as a key metabolite in the coordination of bacterial growth and division. Our results support a model in which pyruvate levels are coupled to Z-ring assembly via an enzyme that actually metabolizes pyruvate, the E1α subunit of pyruvate dehydrogenase. We have shown that this protein localizes over the nucleoid in a pyruvate-dependent manner and may stimulate more efficient Z-ring formation at the cell center under nutrient-rich conditions, when cells must divide more frequently.
Importance: How bacteria coordinate cell cycle processes with nutrient availability and growth is a fundamental yet unresolved question in microbiology. Recent breakthroughs have revealed that nutritional information can be transmitted directly from metabolic pathways to the cell cycle machinery and that this can serve as a mechanism for fine-tuning cell cycle processes in response to changes in environmental conditions. Here we identified a novel link between glycolysis and cell division in Bacillus subtilis. We showed that pyruvate, the final product of glycolysis, plays an important role in maintaining normal division. Nutrient-dependent changes in pyruvate levels affect the function of the cell division protein FtsZ, most likely by modifying the activity of an enzyme that metabolizes pyruvate, namely, pyruvate dehydrogenase E1α. Ultimately this system may help to coordinate bacterial division with nutritional conditions to ensure the survival of newborn cells.
Copyright © 2014 Monahan et al.
Figures



Similar articles
-
Changes in the oligomerization potential of the division inhibitor UgtP co-ordinate Bacillus subtilis cell size with nutrient availability.Mol Microbiol. 2012 Nov;86(3):594-610. doi: 10.1111/mmi.12007. Epub 2012 Sep 10. Mol Microbiol. 2012. PMID: 22931116 Free PMC article.
-
ZapE is a novel cell division protein interacting with FtsZ and modulating the Z-ring dynamics.mBio. 2014 Mar 4;5(2):e00022-14. doi: 10.1128/mBio.00022-14. mBio. 2014. PMID: 24595368 Free PMC article.
-
A new FtsZ-interacting protein, YlmF, complements the activity of FtsA during progression of cell division in Bacillus subtilis.Mol Microbiol. 2006 Jun;60(6):1364-80. doi: 10.1111/j.1365-2958.2006.05184.x. Mol Microbiol. 2006. PMID: 16796675
-
Bacterial cell division: assembly, maintenance and disassembly of the Z ring.Nat Rev Microbiol. 2009 Sep;7(9):642-53. doi: 10.1038/nrmicro2198. Nat Rev Microbiol. 2009. PMID: 19680248 Review.
-
Bacterial cell division: the mechanism and its precison.Int Rev Cytol. 2006;253:27-94. doi: 10.1016/S0074-7696(06)53002-5. Int Rev Cytol. 2006. PMID: 17098054 Review.
Cited by
-
Disruption of the MreB Elongasome Is Overcome by Mutations in the Tricarboxylic Acid Cycle.Front Microbiol. 2021 Apr 23;12:664281. doi: 10.3389/fmicb.2021.664281. eCollection 2021. Front Microbiol. 2021. PMID: 33968001 Free PMC article.
-
Bacterial Cell Size: Multifactorial and Multifaceted.Annu Rev Microbiol. 2017 Sep 8;71:499-517. doi: 10.1146/annurev-micro-090816-093803. Annu Rev Microbiol. 2017. PMID: 28886685 Free PMC article. Review.
-
Deciphering the metabolism of Lactobacillus delbrueckii subsp. delbrueckii during soy juice fermentation using phenotypic and transcriptional analysis.Appl Environ Microbiol. 2024 Mar 20;90(3):e0193623. doi: 10.1128/aem.01936-23. Epub 2024 Feb 20. Appl Environ Microbiol. 2024. PMID: 38376234 Free PMC article.
-
Growth rate and cell size: a re-examination of the growth law.Curr Opin Microbiol. 2015 Apr;24:96-103. doi: 10.1016/j.mib.2015.01.011. Epub 2015 Feb 5. Curr Opin Microbiol. 2015. PMID: 25662920 Free PMC article. Review.
-
Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling.Nature. 2020 Feb;578(7796):582-587. doi: 10.1038/s41586-020-1990-9. Epub 2020 Feb 12. Nature. 2020. PMID: 32051588
References
-
- Harry E, Monahan L, Thompson L. 2006. Bacterial cell division: the mechanism and its precision, p 27–94 In Jeon KW, International review of cytology: a survey of cell biology, vol. 253 Academic Press, San Diego, CA. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

