Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9
- PMID: 24825012
- PMCID: PMC4030483
- DOI: 10.1128/mBio.01114-14
Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9
Abstract
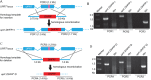
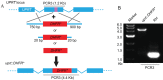
Toxoplasma gondii has become a model for studying the phylum Apicomplexa, in part due to the availability of excellent genetic tools. Although reverse genetic tools are available in a few widely utilized laboratory strains, they rely on special genetic backgrounds that are not easily implemented in natural isolates. Recent progress in modifying CRISPR (clustered regularly interspaced short palindromic repeats), a system of DNA recognition used as a defense mechanism in bacteria and archaea, has led to extremely efficient gene disruption in a variety of organisms. Here we utilized a CRISPR/CAS9-based system with single guide RNAs to disrupt genes in T. gondii. CRISPR/CAS9 provided an extremely efficient system for targeted gene disruption and for site-specific insertion of selectable markers through homologous recombination. CRISPR/CAS9 also facilitated site-specific insertion in the absence of homology, thus increasing the utility of this approach over existing technology. We then tested whether CRISPR/CAS9 would enable efficient transformation of a natural isolate. Using CRISPR/CAS9, we were able to rapidly generate both rop18 knockouts and complemented lines in the type I GT1 strain, which has been used for forward genetic crosses but which remains refractory to reverse genetic approaches. Assessment of their phenotypes in vivo revealed that ROP18 contributed a greater proportion to acute pathogenesis in GT1 than in the laboratory type I RH strain. Thus, CRISPR/CAS9 extends reverse genetic techniques to diverse isolates of T. gondii, allowing exploration of a much wider spectrum of biological diversity.
Importance: Genetic approaches have proven very powerful for studying the biology of organisms, including microbes. However, ease of genetic manipulation varies widely among isolates, with common lab isolates often being the most amenable to such approaches. Unfortunately, such common lab isolates have also been passaged frequently in vitro and have thus lost many of the attributes of wild isolates, often affecting important traits, like virulence. On the other hand, wild isolates are often not amenable to standard genetic approaches, thus limiting inquiry about the genetic basis of biological diversity. Here we imported a new genetic system based on CRISPR/CAS9, which allows high efficiency of targeted gene disruption in natural isolates of T. gondii. This advance promises to bring the power of genetics to bear on the broad diversity of T. gondii strains that have been described recently.
Copyright © 2014 Shen et al.
Figures


References
-
- Dubey JP. 2010. Toxoplasmosis of animals and humans. CRC Press, Boca Raton, FL
-
- Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Dardé ML, Zhu XQ, Ajioka JW, Rosenthal BM, Dubey JP, Sibley LD. 2012. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc. Natl. Acad. Sci. U. S. A. 109:5844–5849. 10.1073/pnas.1203190109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

