Ovarian dendritic cells act as a double-edged pro-ovulatory and anti-inflammatory sword
- PMID: 24825398
- PMCID: PMC5414831
- DOI: 10.1210/me.2013-1400
Ovarian dendritic cells act as a double-edged pro-ovulatory and anti-inflammatory sword
Abstract
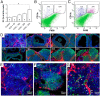
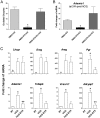
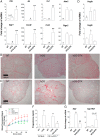
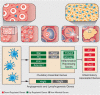
Ovulation and inflammation share common attributes, including immune cell invasion into the ovary. The present study aims at deciphering the role of dendritic cells (DCs) in ovulation and corpus luteum formation. Using a CD11c-EYFP transgenic mouse model, ovarian transplantation experiments, and fluorescence-activated cell sorting analyses, we demonstrate that CD11c-positive, F4/80-negative cells, representing DCs, are recruited to the ovary under gonadotropin regulation. By conditional ablation of these cells in CD11c-DTR transgenic mice, we revealed that they are essential for expansion of the cumulus-oocyte complex, release of the ovum from the ovarian follicle, formation of a functional corpus luteum, and enhanced lymphangiogenesis. These experiments were complemented by allogeneic DC transplantation after conditional ablation of CD11c-positive cells that rescued ovulation. The pro-ovulatory effects of these cells were mediated by up-regulation of ovulation-essential genes. Interestingly, we detected a remarkable anti-inflammatory capacity of ovarian DCs, which seemingly serves to restrict the ovulatory-associated inflammation. In addition to discovering the role of DCs in ovulation, this study implies the extended capabilities of these cells, beyond their classic immunologic role, which is relevant also to other biological systems.
Figures


References
-
- Espey LL. Ovulation as an inflammatory reaction—a hypothesis. Biol Reprod. 1980;22:73–106. - PubMed
-
- Richards JS, Liu Z, Shimada M. Immune-like mechanisms in ovulation. Trends Endocrinol Metab. 2008;19:191–196. - PubMed
-
- Tsafriri A, Lindner HR, Zor U, Lamprecht SA. Physiological role of prostaglandins in the induction of ovulation. Prostaglandins. 1972;2:1–10. - PubMed
-
- Reich R, Kohen F, Slager R, Tsafriri A. Ovarian lipoxygenase activity and its regulation by gonadotropin in the rat. Prostaglandins. 1985;30:581–590. - PubMed
-
- Reich R, Daphna-Iken D, Chun SY, et al. . Preovulatory changes in ovarian expression of collagenases and tissue metalloproteinase inhibitor messenger ribonucleic acid: role of eicosanoids. Endocrinology. 1991;129:1869–1875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

