The Hippo signaling pathway in stem cell biology and cancer
- PMID: 24825474
- PMCID: PMC4197875
- DOI: 10.15252/embr.201438638
The Hippo signaling pathway in stem cell biology and cancer
Abstract
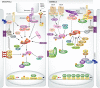
The Hippo signaling pathway, consisting of a highly conserved kinase cascade (MST and Lats) and downstream transcription coactivators (YAP and TAZ), plays a key role in tissue homeostasis and organ size control by regulating tissue-specific stem cells. Moreover, this pathway plays a prominent role in tissue repair and regeneration. Dysregulation of the Hippo pathway is associated with cancer development. Recent studies have revealed a complex network of upstream inputs, including cell density, mechanical sensation, and G-protein-coupled receptor (GPCR) signaling, that modulate Hippo pathway activity. This review focuses on the role of the Hippo pathway in stem cell biology and its potential implications in tissue homeostasis and cancer.
Keywords: Hippo pathway; YAP; cancer; regeneration; stem cell.
© 2014 The Authors.
Figures



References
-
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. - PubMed
-
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. - PubMed
-
- Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. - PubMed
-
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

