Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder
- PMID: 24825486
- PMCID: PMC4127124
- DOI: 10.1111/add.12608
Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder
Abstract
Background and aims: No effective pharmacotherapy for methamphetamine (MA) use disorder has yet been found. This study evaluated sustained-release methylphenidate (MPH-SR) compared with placebo (PLA) for treatment of MA use disorder in people also undergoing behavioral support and motivational incentives.
Design: This was a randomized, double-blind, placebo-controlled design with MPH-SR or PLA provided for 10 weeks (active phase) followed by 4 weeks of single-blind PLA. Twice-weekly clinic visits, weekly group counseling (CBT) and motivational incentives (MI) for MA-negative urine drug screens (UDS) were included.
Setting: Treatment sites were in Los Angeles, California (LA) and Honolulu, Hawaii (HH), USA.
Participants: A total of 110 MA-dependent (via DSM-IV) participants (LA = 90; HH = 20).
Measurements: The primary outcome measure is self-reported days of MA use during the last 30 days of the active phase. Included in the current analyses are drug use (UDS and self-report), retention, craving, compliance (dosing, CBT, MI), adverse events and treatment satisfaction.
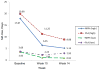
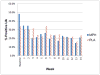
Findings: No difference was found between treatment groups in self-reported days of MA use during the last 30 days of the active phase (P = 0.22). In planned secondary outcomes analyses, however, the MPH group had fewer self-reported MA use days from baseline through the active phase compared with the PLA group (P = 0.05). The MPH group also had lower craving scores and fewer marijuana-positive UDS than the PLA group in the last 30 days of the active phase. The two groups had similar retention, other drug use, adverse events and treatment satisfaction.
Conclusions: Methylphenidate may lead to a reduction in concurrent methamphetamine use when provided as treatment for patients undergoing behavioral support for moderate to severe methamphetamine use disorder, but this requires confirmation.
Keywords: Methamphetamine; RCT; methamphetamine use disorders; methylphenidate; randomized clinical trial; treatment.
© 2014 Society for the Study of Addiction.
Figures


Comment in
-
Ling et al.'s 'Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder'.Addiction. 2015 May;110(5):875-6. doi: 10.1111/add.12885. Epub 2015 Mar 23. Addiction. 2015. PMID: 25808040 Free PMC article. No abstract available.
References
-
- United Nations Office on Drugs and Crime. [Accessed September 2013];World Drug Report 2013. at: http://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf.
-
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658.
-
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162–70. - PubMed
-
- Newton TF, Roache JD, De La Garza R, II, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31(7):1537–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

