Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells
- PMID: 24825548
- PMCID: PMC4120246
- DOI: 10.1016/j.yjmcc.2014.05.001
Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells
Abstract
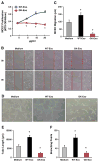
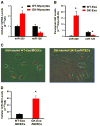
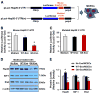
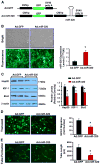
Exosomes, nano-vesicles naturally released from living cells, have been well recognized to play critical roles in mediating cell-to-cell communication. Given that diabetic hearts exhibit insufficient angiogenesis, it is significant to test whether diabetic cardiomyocyte-derived exosomes possess any capacity in regulating angiogenesis. In this study, we first observed that both proliferation and migration of mouse cardiac endothelial cells (MCECs) were inhibited when co-cultured with cardiomyocytes isolated from adult Goto-Kakizaki (GK) rats, a commonly used animal model of type 2 diabetes. However, GK-myocyte-mediated anti-angiogenic effects were negated upon addition of GW4869, an inhibitor of exosome formation/release, into the co-cultures. Next, exosomes were purified from the myocyte culture supernatants by differential centrifugation. While exosomes derived from GK myocytes (GK-exosomes) displayed similar size and molecular markers (CD63 and CD81) to those originated from the control Wistar rat myocytes (WT-exosomes), their regulatory role in angiogenesis is opposite. We observed that the MCEC proliferation, migration and tube-like formation were inhibited by GK-exosomes, but were promoted by WT-exosomes. Mechanistically, we found that GK-exosomes encapsulated higher levels of miR-320 and lower levels of miR-126 compared to WT-exosomes. Furthermore, GK-exosomes were effectively taken up by MCECs and delivered miR-320. In addition, transportation of miR-320 from myocytes to MCECs could be blocked by GW4869. Importantly, the exosomal miR-320 functionally down-regulated its target genes (IGF-1, Hsp20 and Ets2) in recipient MCECs, and overexpression of miR-320 inhibited MCEC migration and tube formation. GK exosome-mediated inhibitory effects on angiogenesis were removed by knockdown of miR-320. Together, these data indicate that cardiomyocytes exert an anti-angiogenic function in type 2 diabetic rats through exosomal transfer of miR-320 into endothelial cells. Thus, our study provides a novel mechanism underlying diabetes mellitus-induced myocardial vascular deficiency which may be caused by secretion of anti-angiogenic exosomes from cardiomyocyes.
Keywords: Cardiomyocytes; Exosomes; Myocardial angiogenesis; Type 2 diabetes; miR-320.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None.
Figures



Comment in
-
Diabetes, microRNAs and exosomes: Les liaisons dangereuses.J Mol Cell Cardiol. 2014 Sep;74:196-8. doi: 10.1016/j.yjmcc.2014.05.014. Epub 2014 May 27. J Mol Cell Cardiol. 2014. PMID: 24874423 No abstract available.
References
-
- Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev. 2005;1:59–63. - PubMed
-
- Cohen G, Riahi Y, Alpert E, Gruzman A, Sasson S. The roles of hyperglycaemia and oxidative stress in the rise and collapse of the natural protective mechanism against vascular endothelial cell dysfunction in diabetes. Arch Physiol Biochem. 2007;113:259–67. - PubMed
-
- Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92:1037–45. - PubMed
-
- Heather LC, Clarke K. Metabolism, hypoxia and the diabetic heart. J Mol Cell Cardiol. 2011;50:598–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

