Resolving Salmonella infection reveals dynamic and persisting changes in murine bone marrow progenitor cell phenotype and function
- PMID: 24825601
- PMCID: PMC4209805
- DOI: 10.1002/eji.201344350
Resolving Salmonella infection reveals dynamic and persisting changes in murine bone marrow progenitor cell phenotype and function
Abstract
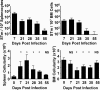
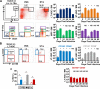
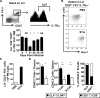
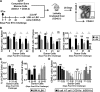
The generation of immune cells from BM precursors is a carefully regulated process. This is essential to limit the potential for oncogenesis and autoimmunity yet protect against infection. How infection modulates this is unclear. Salmonella can colonize systemic sites including the BM and spleen. This resolving infection has multiple IFN-γ-mediated acute and chronic effects on BM progenitors, and during the first week of infection IFN-γ is produced by myeloid, NK, NKT, CD4(+) T cells, and some lineage-negative cells. After infection, the phenotype of BM progenitors rapidly but reversibly alters, with a peak ∼ 30-fold increase in Sca-1(hi) progenitors and a corresponding loss of Sca-1(lo/int) subsets. Most strikingly, the capacity of donor Sca-1(hi) cells to reconstitute an irradiated host is reduced; the longer donor mice are exposed to infection, and Sca-1(hi) c-kit(int) cells have an increased potential to generate B1a-like cells. Thus, Salmonella can have a prolonged influence on BM progenitor functionality not directly related to bacterial persistence. These results reflect changes observed in leucopoiesis during aging and suggest that BM functionality can be modulated by life-long, periodic exposure to infection. Better understanding of this process could offer novel therapeutic opportunities to modulate BM functionality and promote healthy aging.
Keywords: Bacterial Infection; Bone Marrow; Leucopoiesis; Progenitor; Salmonella.
© 2014 The Authors. European Journal of Immunology published by Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. - PubMed
-
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008;9:115–128. - PubMed
-
- Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann. N. Y. Acad. Sci. 2007;1106:41–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

