Pericyazine for schizophrenia
- PMID: 24825770
- PMCID: PMC11023599
- DOI: 10.1002/14651858.CD007479.pub2
Pericyazine for schizophrenia
Abstract
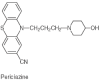
Background: Pericyazine is a 3-cyano-10 (3-4'-hydroxypiperidinopropyl) phenothiazine. It is overall pharmacologically similar with chlorpromazine, though particularly sedating. Dopamine receptor subtype analysis has not been performed for pericyazine, but the drug appears to induce greater noradrenergic than dopaminergic blockade. Compared to chlorpromazine, pericyazine reportedly has more potent antiemetic, antiserotonin, and anticholinergic activity.
Objectives: To evaluate the clinical effects and safety of pericyazine in comparison with placebo, typical and atypical antipsychotic agents and standard care for people with schizophrenia.
Search methods: We searched the Cochrane Schizophrenia Group Trials Register (February 2013) which is based on regular searches of CINAHL, EMBASE, MEDLINE and PsycINFO. We inspected references of all identified studies for further trials.
Selection criteria: All relevant randomised controlled trials focusing on pericyazine for schizophrenia and other types of schizophrenia-like psychoses (schizophreniform and schizoaffective disorders). We excluded quasi-randomised trials.
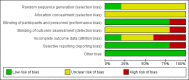
Data collection and analysis: Data were extracted independently from included papers by at least two review authors. Risk ratios (RR) and 95% confidence intervals (CI) of homogeneous dichotomous data were calculated. We assessed risk of bias for included studies and used GRADE to judge quality of evidence.
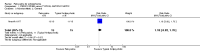
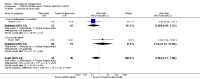
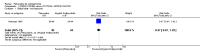
Main results: We could only include five studies conducted between 1965 and 1980. Most of the included studies did not report details of randomisation, allocation concealment, details of blinding and we could not assess the impact of attrition due to poor reporting.For the primary outcome of Global state - not improved, the confidence interval was compatible with a small benefit and increased risk of not improving with pericyazine compared with typical antipsychotics (2 RCTs, n = 122, RR 1.24 CI 0.93 to 1.66, very low quality of evidence) or atypical antipsychotics (1 RCT, n = 93, RR 0.97 CI 0.67 to 1.42, very low quality of evidence).When compared with typical antipsychotics relapse was only experienced by one person taking pericyazine (1 RCT, n = 80, RR 2.59 CI 0.11 to 61.75, very low quality of evidence).Pericyazine was associated with more extrapyramidal side effects than typical antipsychotics (3 RCTs, n = 163, RR 0.52 CI 0.34 to 0.80, very low quality of evidence) and atypical antipsychotics (1 RCT, n = 93, RR 2.69 CI 1.35 to 5.36, very low quality of evidence).The estimated risk of leaving the study early for specific reasons was imprecise for the comparisons of pericyazine with typical antipsychotics (2 RCTs, n = 71, RR 0.46 CI 0.11 to 1.90, very low quality of evidence), and with atypical antipsychotics (1 RCT, n = 93, RR 0.13 CI 0.01 to 2.42, very low quality of evidence).
Authors' conclusions: On the basis of very low quality evidence we are unable to determine the effects of pericyazine in comparison with typical or atypical antipsychotics for the treatment of schizophrenia. However, there is some evidence that pericyazine may be associated with a higher incidence of extrapyramidal side effects than other antipsychotics, and again this was judged to be very low quality evidence. Large, robust studies are still needed before any firm conclusions can be drawn.
Conflict of interest statement
None known.
Figures





Update of
References
References to studies included in this review
Ananth 1977 {published data only}
-
- Ananth JV, Ban TA. A Standard‐controlled clinical study with propericiazine in schizophrenic patients. Psychopharmacology Bulletin 1977;13(3):19‐20. - PubMed
Grant 1965 {published data only}
-
- Grant B, Tonks CM. The relative value of pericyazine and chlorpromazine in disturbed chronic schizophrenics. Proceedings of the Leeds Symposium on Behavioural Disorders. 1965:115‐20.
Iwanaga 1980 {published data only}
-
- Iwanaga M, Akazawa S, Abe T. Clinical evaluation of zetopine in chronic schizophrenia: a multi‐institutional double blind study in comparison with propericiazine. Clinical Psychiatry [Seishin Igaku] 1980;9(8):873‐91.
Leitch 1965 {published data only}
-
- Leitch A. A controlled trial in schizophrenia and allied states with pericyazine. Proceedings of the Leeds Symposium on Behavioural Disorder. 1965:121‐8.
Rasch 1966 {published data only}
-
- Rasch PJ. Treatment of disorders of charater and schizophrenia by pericyazine (Neulactil). Acta Psychiatrica Scandinavica Supplementum 1966;191:200‐15. - PubMed
References to studies excluded from this review
Ananth 1972 {published data only}
-
- Ananth JV, Salib M, Ban TA, Lehmann HE. Propericiazine in psychiatric emergencies. A controlled comparative study. Canadian Psychiatric Association Journal 1972;17(2):143‐5. - PubMed
Barker 1969 {published data only}
-
- Barker JC, Miller M. A double blind comparative trial of pericyazine and thioridazine in chronic schizophrenia. British Journal of Psychiatry 1969;115(519):169‐72. - PubMed
Denef 1971 {published data only}
-
- Denef P, Baro F, Lommel R, Dom R. Treatment of chronic psychotic patients with high doses of propericiazine. Proceedings of the 5th World Congress of Psychiatry. 1971:262‐3.
Deutsch 1971 {published data only}
-
- Deutsch M, Ananth JV, Ban TA. A clinical study with propericiazine in chronic psychotic patients. Current Therapeutic Research (Clinical and Experimental) 1971;13(6):353‐8. - PubMed
Heller 1965 {published data only}
-
- Heller GC, Mather MD. A new phenothiazine in the treatment of psychotic behaviour problems. Proceedings of the Leeds Symposium on Behavioural Disorder. 1965:80‐8.
Nishikawa 1984 {published data only}
-
- Nishikawa T, Tsuda A, Tanaka M, Hoaki Y, Koga I, Uchida Y. Prophylactic effect of neuropletics in symptom free schizophrenics ‐ a comparative dose‐response study of haloperidol and propericiazine. Psychopharmacology 1984;82(3):153‐6. - PubMed
St Jean 1967 {published data only}
-
- Jean A, Sterlin C, Noe W, Ban TA. Clinical studies with propericiazine. Diseases of the Nervous System 1967;28(8):526‐31. - PubMed
Weir 1968 {published data only}
-
- Weir TW, Kemohan GA, MacKay DN. The use of pericyazine and chlorpromazine with disturbed mentally subnormal patients. British Journal of Psychiatry 1968;114:111‐2. - PubMed
Additional references
Altman 1996
Astrup 1965
-
- Astrup C, Clemm F. Clinical and experimental studies of pericyazine in schizophrenic patients. Proceedings of the Leeds Symposium on Behavioural Disorders. Leeds, 1965:144‐50.
Ban 1965
-
- Ban TA. Human pharmacology and systematic clinical studies with a new phenothiazine. Proceedings of the Leeds Symposium on Behavioural Disorders. 1965:240‐53.
Bartlet 1965
-
- Bartlet E. Study of a new phenothiazine in chronic schizophrenia. Proceedings of the Leeds Symposium on Behavioural Disorders. 1965:164‐9.
Becker 1981
-
- Becker RE. Propericiazine: effectiveness against hostility and aggression as compared to chlorpromazine. Current Therapeutic Research 1981;29:925‐8.
Bland 1997
BNF 2008
-
- Joint Formulary Committee. British National Formulary. 55. Pharmaceutical Press, 2008.
Boardman 1965
-
- Boardman RH. Maintenance therapy with pericyazine in chronic psychoses. Proceedings of the Leeds Symposium on Behavioural Disorders. Leeds, 1965:151‐2.
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. - PubMed
Carpenter 1994
-
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. - PubMed
Chanoit 1962
-
- Chanoit P, Deshaies G, Mignot P. Une indication particuliere du 8909 R.P: Les troubles du caractere. Comptes rendus du congres de psychiatrie et de neurologie de langue Francaise. 1962:430‐62.
Chanoit 1964
-
- Chanoit P, Deshaics G. Etude clinique d'un nouveau neuroleptiquc: Le pericyazine. Presse Médicale 1964;71:339.
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Abstracts of 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Deshaies 1962
-
- Deshaies G. The clinical effects of RP‐8909 [Action cliniques du RP‐8909]. Enccphalc 1962;51:602‐7. - PubMed
Divine 1992
-
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal Epidemiology 2002;31:140‐9. - PubMed
Fortinash 2000
-
- Fortinash KM, Holoday‐Worret PA. Psychiatric Mental Health Nursing. London: Mosby, 2000.
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. - PubMed
Grant 1965 a
-
- Grant B, Tonks CM. The relative value of pericyazine and chlorpromazine in disturbed chronic schizophrenics. Proceedings of the Leeds Symposium on Behavioural Disorders. Leeds, 1965:114‐20.
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Higgins 2003
Higgins 2005
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]The Cochrane Collaboration, 2005. Cochrane Handbook for Systematic Reviews of Interventions 2005.
Higgins 2006
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006] The Cochrane Collaboration, 2006. Cochrane Handbook for Systematic Reviews of Interventions. ., 2006.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
Hutton 2009
-
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. - PubMed
Jenner 1970
-
- Jenner AF. Pericyazine in clinical practice. Laval Medical 1970;41(6):796‐804. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986.
Leucht 2005
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales ‐ a major source of bias in randomised controlled trials of treatments for schizophrenia?. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285(15):1987‐91. - PubMed
Nishikawa 1989
-
- Nishikawa T, Tanaka M, Tsuda A, Koga I, Uchida Y. Prophylactic effects of neuroleptics in symptom‐free schizophrenics: a comparative dose‐response study of timiperone and sulpiride. Biological Psychiatry 1989;25(7):861‐6. [MEDLINE: ] - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812.
Rodgers 1996
-
- Rodgers RJ, Johnson NJT, Champion AJ, Mills S. Modulation of plus‐maze behaviour in mice by preferential D3‐receptor agonist 7‐OH‐DPAT. Pharmacology, Biochemistry and Behavior 1996;54:79‐84. - PubMed
Rust 1989
-
- Rust J, Golombok S. Modern Psychmetrics. London: Routledge, 1989.
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Schünemann 2008
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83.
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
Xia 2009
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

