Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors
- PMID: 24825818
- PMCID: PMC4199087
- DOI: 10.2310/7290.2014.00015
Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors
Abstract
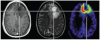
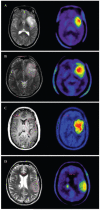
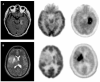
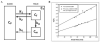
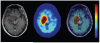
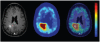
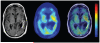
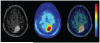
Positron emission tomography (PET) is an imaging technology that can detect and characterize tumors based on their molecular and biochemical properties, such as altered glucose, nucleoside, or amino acid metabolism. PET plays a significant role in the diagnosis, prognostication, and treatment of various cancers, including brain tumors. In this article, we compare uptake mechanisms and the clinical performance of the amino acid PET radiotracers (l-[methyl-11C]methionine [MET], 18F-fluoroethyl-tyrosine [FET], 18F-fluoro-l-dihydroxy-phenylalanine [FDOPA], and 11C-alpha-methyl-l-tryptophan [AMT]) most commonly used for brain tumor imaging. First, we discuss and compare the mechanisms of tumoral transport and accumulation, the basis of differential performance of these radioligands in clinical studies. Then we summarize studies that provided direct comparisons among these amino acid tracers and to clinically used 2-deoxy-2[18F]fluoro-d-glucose [FDG] PET imaging. We also discuss how tracer kinetic analysis can enhance the clinical information obtained from amino acid PET images. We discuss both similarities and differences in potential clinical value for each radioligand. This comparative review can guide which radiotracer to favor in future clinical trials aimed at defining the role of these molecular imaging modalities in the clinical management of brain tumor patients.
Figures
References
-
- Reddy K, Westerly D, Chen C. MRI patterns of T1 enhancing radiation necrosis versus tumour recurrence in high-grade gliomas. J Med Imaging Radiat Oncol. 2013;57:349–55. doi:10.1111/j.1754-9485.2012.02472.x. - PubMed
-
- Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30:552–8. doi:10.3174/ajnr.A1377. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

